Doctoral student Kirika Ueda from the Graduate School of Science at Nagoya University received the "L'Oreal-UNESCO Women in Science Award of Encouragement Japan
 Kirika Ueda, doctoral student at the Graduate School of Science, Nagoya University, received the "L'Oreal-UNESCO Women in Science Award of Encouragement Japan." (Fig 1). This prize was designed to encourage excellent women doctoral students who have potential as future leaders. The prize is funded by Nihon L'Oreal with the cooperation of UNESCO. Nihon L'Oreal is the Japanese manufacturing arm of the L'Oreal group, which is the world's largest cosmetic manufacturer. Only each two researchers in the Life Sciences and Material Sciences are selected as the prizewinners each year.
Kirika Ueda, doctoral student at the Graduate School of Science, Nagoya University, received the "L'Oreal-UNESCO Women in Science Award of Encouragement Japan." (Fig 1). This prize was designed to encourage excellent women doctoral students who have potential as future leaders. The prize is funded by Nihon L'Oreal with the cooperation of UNESCO. Nihon L'Oreal is the Japanese manufacturing arm of the L'Oreal group, which is the world's largest cosmetic manufacturer. Only each two researchers in the Life Sciences and Material Sciences are selected as the prizewinners each year.
Ueda received the prize in Material Sciences for her work in the "development of a new catalyst for biaryls and heterobiaryls, and its application to pharmacologically-active and functional organic materials." Biaryls and heterobiaryls are chemical compounds that include a planar carbon ring system, such as benzene (Fig. 2).
Many pharmaceutical compounds and electronic materials contain biaryls and heterobiaryls in their structures. Coupling reactions between aromatic rings are very important for the production of such materials. Aromatic coupling reactions, however, are not easy to achieve. The reactions are promoted by catalysts, which are chemical compounds that lower the barrier to the reaction and are not themselves consumed during the course of the reaction. In some cases, the production of a target material can require many catalytic reactions, which incur large production costs. To efficiently carry out a total synthesis, it is frequently desirable to reduce the number of catalytic reactions. Regioselective coupling reactions are also important for the synthesis of useful products, and the development of catalysts that can efficiently promote such reactions under mild conditions remains a goal among chemists.
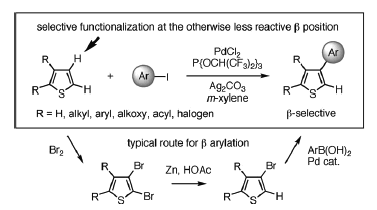
Ueda has risen to challenge posed by this difficult problem. Her research program is ideal chemical synthesis through C-H transformation. The direct C-H bond arylation reaction of arenes or heteroarenes has become an emerging methodology for making privileged biaryls and heterobiaryls. She has succeeded in developing a new catalyst for a couping reaction of thiophenes (Fig. 3) with iodoarenes. This catalyst can directly and selectively functionalize the β-carbones of thiophene ring (Fig. 4). Previous synthetic approaches have relied on three consecutive catalyzed reactions. Ueda also shows that the catalytic system can apply to synthesize the compound which is a potential drug candidate for treatment of Alzheimer's disease and Tetraarylthiophenes which may be used as an organic material.
Upon acceping the prize, Ueda stated "I am very honored to receive this prize, which is only granted to two researchers in the field of Material Sciences. There are few women researchers, it is my aspiration to become a leader in my field, and I hope that my success will encourage women to enter the career path presented by scientific research. I will continue to do my best research in support of this hope."And Ueda told her respect researchers, "I respect two researchers. One of them is Kenichiro Itami, who is my doctoral research aviser. Under his guidance and through his engaging lessons in chemistry, I have grown to recognize that chemistry is relevant to pursuits in nearly all scientific disciplines. I decided to pursue chemical research based on this inspiration. Dr. Itami holds my utmost respect as a researcher and educator. I would also respect Akira Suzuki, winner of the Nobel Prize in Chemistry in 2010 for the development of the Suzuki Coupling reaction. His research has contributed tremendously to our society by facilitating the synthesis of many drugs. As Dr. Suzuki has done in his research, I, too, will advance my research toward the goal of developing useful technologies through synthetic chemistry."Commenting on her career path and future work, Ueda said "By developing catalysts, I will advance our fundamental understanding of chemical processes and push forward applications-based research toward development of useful chemical products. Along my career path, I intend to collaborate internationally with other chemistry groups. I have traveled to Germany to study and conduct research in chemistry, which afforded me many opportunities to engage in stimulating discussions with the wider chemistry community. Communicating with chemists internationally will advance my career goals, and I hope that my research will be applied all over the world."
Reference
- Kirika Ueda, Shuichi Yanagisawa, Junichiro Yamaguchi, and Kenichiro Itami,"A General Catalyst for the b-Selective C-H Bond Arylation of Thiophenes with Iodoarenes", vol.49, pp.8946-8949, Angew. Chem. Int. Ed., 2010.