Features
Features
Solving a 140-year-old riddle
- ツイート
- 2011/01/27
Researchers at Nagoya University's GCOE for Advanced Systems Biology are developing new imaging techniques to observe a long sought-after key chemical mechanism behind flowering plant pollination.

Tetsuya Higashiyama, a professor of plant biology and a member of the Advanced Systems Biology GCOE at Nagoya University, is a man who thinks small. In his office in the Graduate School of Science, he leaves through a pile of images of magnified plant cells imagining himself as a minute scientist or explorer able to pull out and examine their various organelles at will. "The world of the cell as we see it through the microscope is so clear," he says. "But there is a big gap between observation and manipulation. It's not until you try to get hold of some of these structures that you actually realize how small they are. My dream is to be able to manipulate whatever parts of the cell I want, and analyze them in whatever way I want." Higashiyama is softly spoken but unmistakably determined. "We are aiming to examine cells and molecules of living material with complete control under the microscope," he says.
Bold visions
Such an ambition would be challenging enough, but for Higashiyama it is only a stepping stone en route to the larger goal of establishing the discipline of 'live cell biology'--the real-time analysis of intercellular signaling in multicellular organisms. It is Higashiyama's hope that such an achievement would elevate Nagoya University to become a world-class center for cellular imaging. It's a bold vision with far-reaching implications, and one that has caught the imagination of the Exploratory Research for Advanced Technology (ERATO) program, a major Japanese government scientific research initiative that has granted \1.8 billion over five years to help support the development of new technologies that can be used immediately by other researchers in the Advanced Live Imaging Center GCOE. The ERATO funds will allow Higashiyama to employ and equip a new team of around 20 researchers who will investigate projects in embryogenesis in a new, purpose-built center at Nagoya University. At the same time, he will still maintain his current group of 20, which he leads in his capacity as a principal investigator in the Advanced Systems Biology GCOE. Becoming the head of a major, high-profile research center and shouldering the responsibility that comes with such a position would be a sufficiently daunting challenge for much older researchers but Higashiyama, who although still only 39 has already been a full professor at Nagoya University or four years, is unfazed: "It was wonderful to get this chance," he says. "This work needs a lot of talented researchers and this position will help me get them. Nagoya University is very supportive of young researchers and I was very fortunate that when I got the ERATO project they were able to help in developing the techniques for live cell analysis that will be used in our research. I'm very happy."
Source of attraction
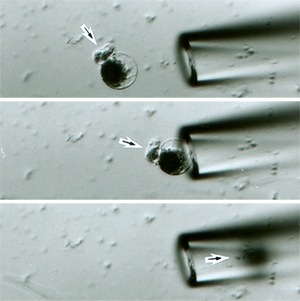
Higashiyama, who was awarded his PhD from the prestigious University of Tokyo in 1999 and where he furthered his career as a research associate for over eight years, moved to Nagoya University as full professor in 2007 at the age of just 35 with his wife Narie Sasaki, who is also a cell biologist and currently an associate professor in his group. His work focuses on real-time observation of the double fertilization reproduction mechanism of flowering plants. In this, pollen is introduced into the pistil of a flower by a pollen tube that extends into the egg apparatus embedded in an ovule--an unfertilized seed--to deliver two sperm cells, one of which fertilizes the egg cell while the other joins with two polar nuclei to form the endosperm or nutrient sac that supplies nourishment to the developing embryo. Although the various stages of this process on the macroscopic level are well understood, the precise details of the mechanism on the molecular level, including the origin and nature of the chemical attractant believed to guide the extension of the pollen tube toward the ovule, had eluded explanation for over 140 years. The difficulty of studying this process was compounded by the fact that fertilization takes place deep inside the flower head and so cannot be observed directly. To obviate this problem, Higashiyama studied the wishbone flower (Torenia fournieri), which has an egg apparatus that protrudes outside of the ovule itself, allowing the fertilization process to be examined more readily. Using specially developed real-time imaging techniques, his team observed the extension of a single pollen tube from a pollen grain attached to the protruding egg apparatus of the ovule. The pollen tube was observed to penetrate one of two 'synergid' cells located near the egg cell. This synergid cell then ruptured as the sperm cells were discharged from the pollen tube. Using the technique of laser ablation, Higashiyama selectively disabled parts of the central area of the embryo sac and observed that the pollen tube was not attracted to the egg cell only when both synergid cells were knocked out. "When we removed the synergid cells, the attraction ceased completely," says Higashiyama. "But interestingly, we found out that if we left one of the two cells intact in the embryo sac, that was still enough to maintain the attraction".
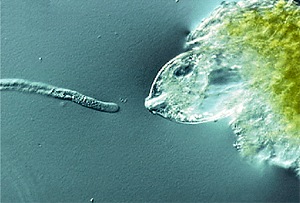
Having confirmed the synergid cells as the source of the pollen tube attractant, the next step was to identify the chemical species responsible for the attraction. Higashiyama was able to isolate from the synergid cells a cocktail of polypeptide molecules containing a high proportion of the amino acid cysteine. The isolation of these polypeptides was itself a painstaking exercise, involving harvesting of synergid cells under the microscope, amplification of the synergid cell complementary DNA using a technique known as the long-distance polymerase chain reaction, and finally some recombinant genetic engineering. What Higashiyama found on closer inspection of two of these polypeptides--collectively dubbed LUREs by the team--were the long sought-after chemical attractants that guide the extension of the pollen tube. The research team confirmed that these LURE polypeptides were the sole attractants by placing gelatin beads bearing the purified proteins in the path of the pollen tube, and by injecting the proteins directly into the path of the pollen tube using a laser-assisted ultrahigh-pressure microinjector developed and patented by Higashiyama. "By depositing beads into the path of the pollen tube we found that as few as 1,000 molecules of LUREs were sufficient to change the direction in which the pollen tube moves," explains Higashiyama.
Questions of efficiency
Building on this experience, the team has begun studying the double fertilization process of Arabidopsis, a widely studied plant species having reproductive equipment buried within the structure of the pistil. Using live cell imaging, the team managed for the first time to distinguish between the two sperm cells that are injected into an embryo sac in real time. They are now using the technique to try and determine whether the roles of the sperm cells--fertilization and endosperm creation--are pre-assigned to individual sperm cells. "Reproduction in flowering plants is very efficient and we want to use our techniques to answer fundamental questions about these processes, such as why each pollen tube is guided directly to only one of the fifty or so egg cells contained in the pistil," comments Higashiyama. Beyond this, his ambition is to examine mechanisms of organ formation by visualizing other important signaling molecules such as auxin, an important plant hormone, and morphogens associated with the growth of animal embryos. This has the promise of providing clues to answering fundamental questions such as how organ size is regulated in living systems. "We need to develop more sophisticated and better techniques to examine the effect of signaling molecules in plant tissues. The invention of new visualization and manipulation techniques would be a breakthrough for many fields of both plant and animal biology. Realizing this will be a key aim of our ERATO project," he says.
NU Research
(English)

