Features
Features
Enhanced plant photosynthesis and growth through manipulation of stomatal apertures
- ツイート
- 2017/11/09
Institute of Transformative Bio-Molecules (ITbM) / IAR
Designated Assistant Professor Yin Wang
Source: IAR Letter, vol.15, March 2017
*about IAR: Nagoya University's Institute for Advanced Research was established to produce internationally recognized academic research of the highest caliber, and to contribute to society through the research achievements at the Institute.
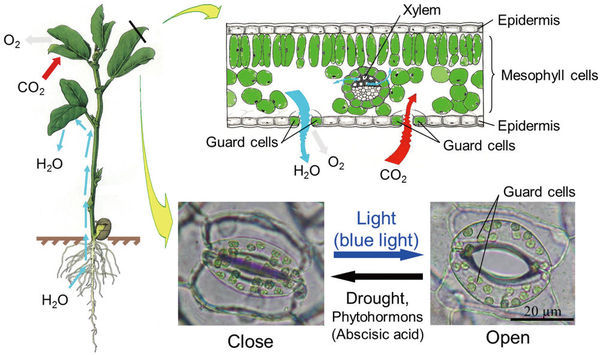
Each stoma (pl. stomata) is controlled by a pair of special cells, named guard cells. Stomata regulate gas exchange between the plant and the atmosphere.
Stomata are microscopic pores surrounded by two guard cells and play an important role in the uptake of carbon dioxide (CO2) for photosynthesis. Here, we showed that overexpression the amount of plasma membrane H+-ATPase in guard cells had a significant effect on light-induced stomatal openings, and therefore enhanced photosynthesis activity and plant growth. Application of this strategy in crops and fuel plants is expected to contribute greatly to the promotion of plant production and a sustainable low-carbon society.
INTRODUCTION
Photosynthesis is a fundamental process that has a close relationship with two critical worldwide problems: global climate change and food shortage. How to improve the efficiency of photosynthesis of land plants to promote carbon dioxide (CO2) absorption and increase crop growth is a hot topic for biologists. Leaves are the primary involved in plant photosynthesis. As the leaf surface is virtually impermeable to air and water, key microscopic pores--known as stomata--provide the major pathways for the diffusion of CO2, O2, and water vapor between the ambient atmosphere and the interior of the leaf. This facilitation of gas exchange by stomatal openings is one of the most essential processes in plant photosynthesis and transpiration.
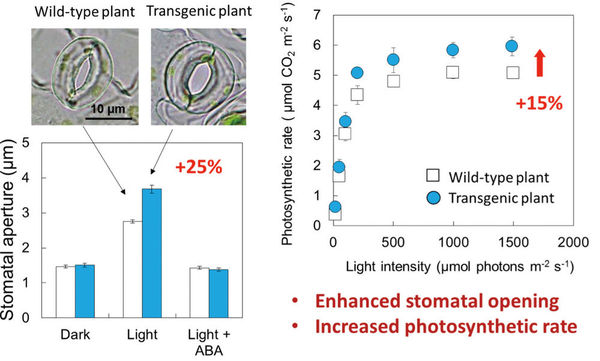
Figure 1: Transgenic plant (plant with overexpressed AHA2) showed larger stomatal apertures and a higher photosynthetic rate than wild-type plants.
Stomata are microscopic pores found on the surface of the leaves, petioles, stem, and other aerial parts of a plant. Each stoma is bound by two specialized cells in the epidermis, called "guard cells," which regulate and control the size of the stomatal aperture (see Figure 1). Light is one of the principal factors that stimulates stomatal opening, and various mechanisms underlie stomatal opening in response to light. Recent studies on Arabidopsis thaliana (a typical model plant) have revealed that the signaling pathway of light induces stomatal opening involving at least three key components-- photoreceptors (phototropins), the plasma membrane H+-ATPase, and inward-rectifying K+ channels (K+in channels) (1) . Therefore, it is expected that the overexpression of those three components in Arabidopsis guard cells could induce stomatal opening to increase photosynthesis and plant growth. However, no previous studies have determined stomatal effects on plant growth by manipulating stomatal apertures via gene regulation in guard cells, perhaps because of the difficulty of balancing the counteracting effects of taking up CO2 while losing water vapor through the stomata (2) .
Overexpression of H+-ATPase in Guard Cells Promotes Light-Induced Stomatal Opening and Enhances the Photosynthetic Rate
Using the Guard Cell 1 (GC1) promoter, we successfully overexpressed PHOT2 (an isoform of the blue-light receptor phototropin), AHA2 (a typical isoform of the plasma membrane H+-ATPase), AKT1 and KAT1 (isoforms of plasma membrane K+in channels) in guard cells, and thereby obtained homo lines with high expression levels. Then, physiological studies on Arabidopsis plants with the overexpressed components were performed. Via isolation of the epidermis of 3- to 4-week-old mature leaves, stomatal apertures were examined by microscope. The results showed that the stomatal apertures of a plant with overexpressed AHA2 opened wider than those of wild-type (WT) plants exposed to light illumination for 2.5 hours, but these stomata closed in darkness just as those in the WT plants (Figure 2). It also demonstrated that the stomata of the plants with overexpressed AHA2 opened more quickly than WT stomata over a period of 30 minutes of illumination. In contrast, the overexpression of PHOT2, AKT1 and KAT1 had no effect on stomatal opening under light conditions. These results indicated that H+-ATPase, not phototropins or K+in channels, is the limiting factor in light-induced stomatal opening, and that increasing the amount of H+-ATPase in guard cells increases the magnitude and speed of stomatal opening (3).
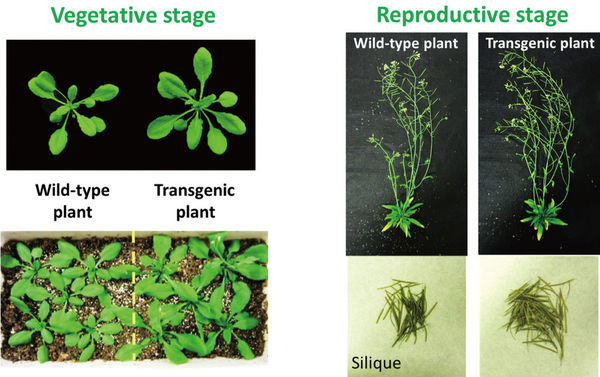
Figure 2: The transgenic plant (plant with overexpressed AHA2) exhibited greater plant growth in both the vegetative stage and the reproductive stage than the wild-type plant.
Then, the stomatal conductance (reflection of the stomatal gas-exchange ability of intact leaves) and photosynthetic activity (photosynthetic rate) of intact leaves of the plants with overexpressed AHA2 were examined in detail by a gas-exchange system. As expected, the stomatal conductance and photosynthetic rate were significantly higher in the plants with overexpressed AHA2 than in the WT plants (Figure 2). To determine whether the higher photosynthetic rate of the plants with overexpressed AHA2 was due to the increased stomatal opening, we examined the response curve between the CO2 assimilation rate and the leaf intercellular CO2 concentration using a gas-exchange system under saturated light conditions. The two curves were almost coincident with each other, indicating that both the Rubisco carboxylation capacity and the electron transport capacity were similar in the WT and transgenic plants, but only the stomatal conductance was greater in the plants with overexpressed AHA2. These results indicate that the enhanced stomatal opening in the plants with overexpressed AHA2 contributed to the increased photosynthetic rate (3).
Overexpression of H+-ATPase in Guard Cells Enhances Plant Productivity
Grown under conditions of sufficient light (about 200 μmol photon m-2 s-1), the plants with overexpressed AHA2 exhibited superiority in plant growth. They produced larger and increased numbers of rosette leaves with approx. 42-63% greater fresh and dry weights than the WT plants in the vegetative stage (25 days old). Moreover, the dry weights of the total flowering stems in the reproductive stage (45 days old), including seeds, siliques, and flowers, of the plants with overexpressed AHA2 were approx. 36-41% greater than those of the WT plants under the same growth conditions. The number of siliques per plant with overexpressed AHA2 was much greater than for the WT plants, although the dry weights of individual siliques from the plants with overexpressed AHA2 were comparable to those from the WT plants (3), more the intention of the husbands, who typically had a larger ideal family size than their wives.
Environmental response of plants with overexpressed H+-ATPase in guard cells
It should be mentioned that the increment of plant growth in the plants with overexpressed AHA2 was obtained under laboratory conditions with proper light, water, temperature, and CO2 concentration. However, in the field, the environmental factors are not always optimal for plant growth, and even worse, certain abiotic and biotic stresses may occur. To investigate these factors, the plants with overexpressed AHA2 were grown under various unfavorable conditions including low light, short daylight hours, and high CO2, and the stomatal apertures and plant growth were subsequently examined. The results showed that the stomatal apertures of the plants with overexpressed AHA2 were still larger than those of the WT plants under all the growing conditions. However, since under low light and high CO2 conditions stomatal limitation did not constitute a key limitation to photosynthesis, the superiority in growth was negated under those two conditions. Only under the short-daylight-hours condition did the plants with overexpressed AHA2 grow better and larger than the WT plants.
Meanwhile, the stress resistance of the plants with overexpressed AHA2 to drought and high temperature (considered as two major abiotic stresses) and pathogens (considered as the major biotic stress) were examined. It is notable that the plants with overexpressed AHA2 showed normal stress resistance, equivalent to the WT plants, under all the abiotic and biotic stresses. These results provide important additional information of how stomatal aperture manipulation biotechnology can be used to promote stomatal opening and enhance plant growth in the field.
Acknowledgements
I would like to express my sincere appreciation to Prof. Kinoshita and the lab members for their advice and help in this work. Special thanks also go to the other co-authors: Dr. Ichiro Terashima, Dr. Ko Noguchi, Dr. Shin-ichiro Inoue and Ms. Natsuko Ono.
References
(1) Shimazaki K., et al., Annu Rev Plant Biol. 58, 219-247 (2014).
(2) Roelfsema M. & Hedrich R., New Phytol. 167, 665-691 (2005).
(3) Wang Y., et al., PNAS, 111, 533-538 (2014).
 |
Yin Wang: Designated Assistant Professor of the Young Leaders Cultivation Program Institute of Transformative Bio-Molecules (ITbM) / Institute of Advanced Research, Nagoya University |
Related Links
- download IAR_letter_Wang.pdf
NU Research
(English)