Amino acids extend the utility of nanoscale quantum dots for bioimaging applications
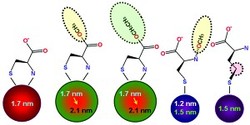
Fig. 1: Using the amino acid cysteine (far left) as a capping agent produces water-soluble quantum dots. Larger or smaller quantum dots can be made by using cysteine derivatives that block amine groups from attaching to the quantum dot surface.
c 2010 Y.-S. Park
To illuminate the complex world of biological systems, scientists require small, stable sources of light. These can be provided by optically active nanoparticles called quantum dots, which are made from semiconducting materials like cadmium selenide (CdSe). A team of researchers from Japan led by Yoshinobu Baba from Nagoya University's FIRST Research Center for Innovative Nanobiodevices has now developed a low-cost, environmentally benign way to synthesize quantum dots that are compatible with biological organisms1.
Quantum dots have great potential as medical imaging agents because of their strong photoluminescence and tunable optical capabilities. In fact, it takes just a small change to the quantum dot size to unleash a spectrum of new emission colors. Eventually, this technology may enable multi-color tracking of concurrent processes in living cells.
Unfortunately, toxic organic liquids are normally used to produce CdSe nanoparticles, and the quantum dot capping agents -- small organic molecules that coat the nanoparticles and stabilize them -- are often not soluble in water. Both of these characteristics present formidable challenges to any bio-related applications.
Baba and his co-workers side-stepped these problems by capping the nanoparticle with an amino acid called cysteine and its derivatives. This method allows the size-selective synthesis of CdSe quantum dots in aqueous solution.
"Amino acid capping molecules are useful for fabricating semiconductor nanoparticles because one or more of their free functional groups are available for further surface modification with biomolecules," explains Yeon-Su Park, one of the lead researchers on the project. Cysteine contains three distinct chemical functional groups that can influence the growth and formation of CdSe nanoparticles: a sulfur-hydrogen unit (SH), a carboxylic acid (CO2H) and an amine group (NH2).
Although previous studies have shown that adding cysteine to cadmium and selenide ions in water produces very stable quantum dots, the separate roles of the functional groups has been unclear. To resolve this uncertainty, the researchers studied nanoparticle formation using a series of cysteine derivatives that systematically interfere with the function of each amino acid.
The experiments revealed that both the SH and NH2 functional groups coordinated directly to the quantum dots, whereas the free CO2H units helped stabilize nanoparticle suspensions. By altering these chemical interactions with the cysteine derivatives, the researchers were able to control the size of the nanoparticles. For example, using capping molecules that prevented NH2 from attaching to the CdSe surface yielded significantly smaller nanoparticles than with the use of pure cysteine (Fig. 1).
The research team's next goal is to synthesize water-soluble nanoparticles that are active in the near-infrared (NIR) spectral region -- a wavelength of light that can penetrate deep into tissue with little loss of intensity. "NIR quantum dots are very promising for cell detection and tracing in our bodies at single molecular level," says Park.
Affiliated Researchers
The Nagoya University affiliated researchers mentioned in this highlight are from the FIRST Research Center for Innovative Nanobiodevices.
Reference
- Park, Y.-S., Dmytruk, A., Dmitruk, I., Kasuya, A., Takeda, M., Ohuchi, N., Okamoto, Y., Kaji, N., Tokeshi, M. & Baba, Y. Size-selective growth and stabilization of small CdSe nanoparticles in aqueous solution. ACS Nano 4, 121-128 (2010). | article