The signaling molecule semaphorin regulates mRNA translation and the actin cytoskeleton by altering the formation of two TOR complexes.
 Semaphorins are signaling molecules, which are proteins that transmit information between cells and regulate cellular activity, that guide nerve fiber formation during development of the nervous system. Due to these important functions, semaphorins have received tremendous attention from researchers studying nerve regeneration. In addition, semaphorins are also involved in suppressing cancer metastases and activating the immune system. In order to develop drugs that regenerate nerves and suppress cancer metastases, it is important to determine the detail mechanisms by which semaphorins induce morphological changes.
Semaphorins are signaling molecules, which are proteins that transmit information between cells and regulate cellular activity, that guide nerve fiber formation during development of the nervous system. Due to these important functions, semaphorins have received tremendous attention from researchers studying nerve regeneration. In addition, semaphorins are also involved in suppressing cancer metastases and activating the immune system. In order to develop drugs that regenerate nerves and suppress cancer metastases, it is important to determine the detail mechanisms by which semaphorins induce morphological changes.
TOR (Target Of Rapamycin) is a pivotal regulator of cell growth, morphogenesis and proliferation. Due to these central functions, TOR is widely studied in cancer and immunology. When complexed with other proteins, TOR performs many cellular functions as highlighted above. However, the mechanisms that regulate the activity of these TOR complexes have not been fully elucidated.
Shin Takagi led a research team from the Graduate School of Science and "Advanced Systems-Biology: Designing The Biological Function" Global COE program at Nagoya University that determined that semaphorins regulate mRNA translation and the actin cytoskeleton by altering the activation state of two TOR complexes.
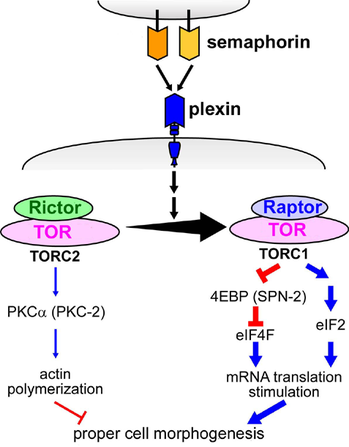
To conduct these studies, this research team used the nematode C. elegans, which is an excellent multicellular animal model due to the efficiency of experimentation. They generated a genetically modified C. elegans that was deficient in semaphorins, and then identified secondary mutations that suppress the defects in semaphorin signaling. As a result, they identified a suppressor gene that was related to TORC2, which is a complex between TOR and the protein Rictor, and determined that there is a relationship between semaphorin signals and TORC2. In order to further examine the relationship between semaphorins and two TOR complexes TORC2 and TORC1, which is composed of TOR and Raptor, they manipulated TORC1- and TORC2-related genes to regulate the activation of TORC1 and TORC2.
Consequently, they showed that semaphorins activate TORC1 but inhibit TORC2 by increasing the amount of the former while decreasing the latter. While activating TORC1 stimulates mRNA translation through two pathways involving eIF2 and eIF4F, inhibiting TORC2 leads to actin depolymerization (Fig. 1). mRNA translation and actin depolymerization are necessary to alter the actin cytoskeleton. mRNA is a "blueprint" that produces cellular components. The cytoskeleton is composed of linear polymers of actin subunits. Therefore, actin depolymerization promotes the destabilization and alteration of the cytoskeleton.
These studies were the first to identify the detail mechanisms in which two TOR complexes relating to various cell activities are regulated by semaphorin signals. Then these findings showed that single signal molecules semaphorins may contribute to various cell activities. It will guide research on TOR and semaphorins.
Takagi explained "We have determined that semaphorin signals regulate two TOR complexes and that this results in changes in the actin cytoskeleton. TORC1 and TORC2 not only contribute to cytoskeletal reorganization but also cancer, cellular senescence and other morphogenetic events. In the future, we will further research how the TOR complexes and semaphorin signals are also involved in these processes. Ultimately, we hope these studies will be highly useful in producing new drugs."
Affiliated Researchers
Graduate School of Science and "Advanced Systems-Biology: Designing The Biological Function" Global COE program
Reference
1. Nukazuka A, Tamaki S, Matsumoto K, Oda Y, Fujisawa H and Takagi S. A shift of the TOR adaptor from Rictor towards Raptor by semaphorin in C.elegans. Nature Communications. (2011 Sep 27.) Volume:2, Article number:484 DOI:doi:10.1038/ncomms 1495.