Highlights
Highlights
Exploring the entire story of plant fertilization using live imaging
- Read in Japanese
- ツイート
- 2015/10/21
- WPI-ITbM
- JST-ERATO Higashiyama Live-Holonics Project
- Institute for Advanced Research
- Assistant Prof. Daisuke Maruyama
- Prof. Tetsuya Higashiyama
Prof. Tetsuya Higashiyama's research groups, WPI-ITbM and JST-ERATO Higashiyama Live-Holonics Project, Nagoya University, and Assistant Prof. Daisuke Maruyama from the Institute for Advanced Research, Nagoya University, have successfully observed the entire plant fertilization process. By examining Arabidopsis following fertilization, they observed the synergid endosperm fusion in real time and explained how function of the synergid cell is stopped in pollen tube attraction.
In general, the cell wall, which surrounds the plant cell, prevents cell-to-cell fusion in plants. However, the findings of this study suggest a new function of the plant cell in cell fusion that will replace the principles that have been previously published in textbooks.
This study was published online in Cell on April 23, 2015.→ Nagoya Universtiy Press Release
Do plants quietly grow where they are rooted? Here we show the dynamic story of plant fertilization as revealed through live imaging.
"A piece of rice or wheat we eat is born as the benefit of the plant fertilization."
Prof. Tetsuya Higashiyama, the vice-director of WPI-ITbM and the project leader of JST-ERATO Higashiyama Live-Holonics Project, Nagoya University, is interested in plant fertilization because of its extreme importance in our lives. His research group has been working to reveal the entire story of plant fertilization using unique and original approaches.
“I wanted to see plant fertilization alive, as it is alive.”
Prof. Higashiyama developed the world’s first live imaging technique in the field of fertilization to explore the entire mechanism of plant fertilization in the living plant (Movie 1 [in Japanese]).
Movie 1. Live imaging technique developed by Prof. Higashiyama, on Science Channel (Copyright JST)
Using this technique, his research group has observed the role of cells following fertilization, the outcomes of which have challenged conventional theory.
*******
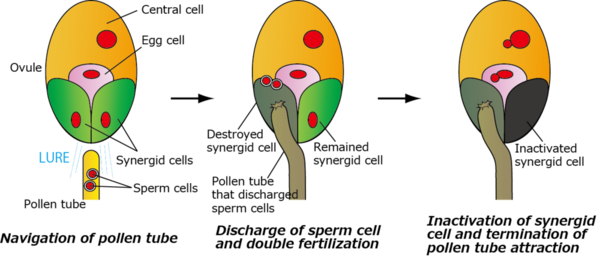
Figure 1 describes the fertilization process in angiosperms.
Figure 1.Double fertilization and inactivation of synergid cell in angiosperms (Figure : by courtesy of Assistant Prof. Maruyama)
When a pollen grain attaches on the tip of the pistil, the pollen forms a pollen tube that grows through the pistil and extends down to the ovule located deep within the pistil. The ovule is the tissue that will later develop into a seed. Inside the ovule, there are seven unique reproductive cells: an egg cell, a central cell, two synergid cells, and three antipodal cells. By releasing pollen tube attractant peptides, LUREs, the two synergid cells can help the pollen tube to navigate its way to the ovule.(Okuda, et al., Nature, 458, 357-361, 2009)
The tip of the pollen tube contains two sperm cells.
The pollen tube discharges these sperm cells into one of the two synergid cells inside the ovule and destroys the receptive synergid. Double fertilization then occurs, whereby one sperm cell fertilizes the egg cell, whereas the other fertilizes the central cell. The fertilized egg and central cells produce the embryo and endosperm, respectively, which form the seed. Interestingly, the other synergid cell termed persistent synergid is immediately inactivated, and terminates the pollen tube attraction.
—To study what causes the cessation of the pollen tube attraction, the research group observed the stage when a seed begins to develop.
“Double fertilization could be a key to stop the pollen tube attraction.”
This idea was first hypothesized, said Assistant Prof. Maruyama. If so, another new pollen tube will be attracted to the ovule if the egg and/or central cells are not fertilized.
He examined a mutant plant that produces defective sperm cells and abolishes both fertilizations with the egg and central cells, and found that 80% of the unfertilized ovule received two pollen tubes. This result demonstrates that the persistent synergid cell keeps its life, unless double fertilization occurs.(Kasahara, et al., Curr. Biol. 22, 1084-1089, 2012)
“Which cell is more important for fertilization, an egg cell or a central cell, then?”
To answer this new question, Assistant Prof. Maruyama developed a mutant that contained only one sperm cell, such that it could fertilize either the egg or central cell.
As a result, despite the single fertilization of the egg cell, the probability of the second pollen tube reaching the ovule was approximately 30%. Similar result was obtained for the single fertilization of the central cell. These data indicated that both the egg and central cells require to be fertilized to inactivate the synergid cell.(Maruyama, et al., Dev. Cell 25, 317-323, 2013)
In this study, Assistant Prof. Maruyama tackled the mystery of how the persistent synergid is inactivated following fertilization.
He examined dynamics of mitochondria labeled with green fluorescent protein (GFP) in the persistent synergid, expecting degeneration of the mitochondoria, the organelles generating most of the energy, play key roles in programmed cell death in animals. Instead of the mitochondrial decay, he unexpectedly observed the mitochondria’s movement from the persistent synergid to large neighboring cell, the endosperm (Movie 2).
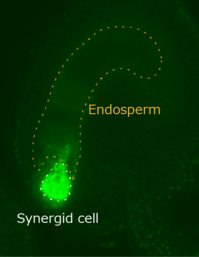
Movie 2.Mitochondria's movement from synergid cell to endosperm
(Movie and Figure : by courtesy of Assistant Prof. Maruyama)
“There may be a big hole that allows the mitochondrial migration.”
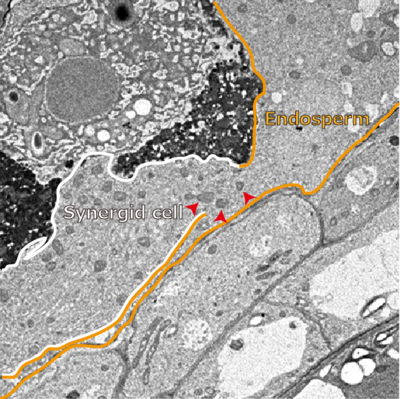
Assistant Prof. Maruyama checked the boundary between synergid cell and the endosperm by electron microscopy and found that a big hole in the fertilized ovules but not in unfertilized ovules. This new discovery demonstrated that the synergid cell and endosperm fuse following fertilization.
Figure 2.Observation by electronic microscopy. Between the synergid cell and endosperm, there was a big hole (shown by arrows).(Figure : by courtesy of Assistant Prof. Maruyama)
An efflux of the pollen tube attractant precursor from the synergid cell into the voluminous endosperm via this hole induces drastic loses of the synergid cell function. Furthermore, nucleus of the persistent synergid cell undergoes mitosis but fails to segregate its chromosomes; consequently, it is collapsed with the endosperm nucleus to proliferate. The persistent synergid cell finally loses its identity by this selective nuclear elimination (Movie 3).
Movie 3.Nuclear elimination
(Movie : by courtesy of Assistant Prof. Maruyama)
In this study, the researchers not only clarified the entire inactivation mechanism for synergid cells, but also succeeded in observing how cell fusion occurs in plants.
The synergid-endosperm fusion observed using by a live imaging technique during double fertilization is “unexpected.” It challenges the conventional belief that, unlike animal cells, plant cells are surrounded by stiff cell walls and raises the possibility of other instances of cell fusion in plants.
*******
This study revealed the entire mechanism of plant fertilization and explained why the pollen tube ceases to grow toward the fertilized ovule. Nature intended for plants to be able to produce seeds from only a few grains of pollen.
“Further development of this finding could allow us to increase plant productivity in the future.”
Assistant Prof. Maruyama expects that the knowledge on this cell fusion mechanism will aid the development of technology in the future.
An understanding of the surprising abilities of plants could indicate a new way to resolve agricultural issues in the future.
(Ayako Umemura)
Researchers featured in this article
Dr. Tetsuya Higashiyama【Professor, Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University】
Dr. Higashiyama graduated from the Department of Biological Sciences, Faculty of Science, University of Tokyo, Japan, in 1994. He completed his doctorate degree in science at the University of Tokyo in 1999. He was awarded a research fellowship for young scientists at the University of Tokyo in 1998, and became an assistant professor at the Graduate School of Science at the university in 1999. Since 2007, he has worked as a professor at the Graduate School of Science, Nagoya University. Between 2008 and 2011, Dr. Higashiyama worked as a PRESTO researcher. He has been working as the project leader for ERATO Higashiyama Live-Holonics Project since 2010, and as the vice-director at WPI-ITbM since 2013.
***
Dr. Higashiyama admires his mentor, Prof. Tsuneyoshi Kuroiwa (Emeritus Professor, University of Tokyo), whose educational policy was “how to make a decoy sweet fish swim freely in many of chances, without notifying it is actually controlled...” Dr. Higashiyama says that he was the sweet fish decoy, but thankfully was able to develop his original researching style because of this policy.
Dr. Higashiyama’s research findings have been used to update school textbooks. He is continuing this research as he enjoys being the first person to discover things, and I expect further achievements from him in the near future (by AU)
Dr. Daisuke Maruyama【YLC Assistant Professor, Institute for Advanced Research, Nagoya University】
Dr. Maruyama graduated from the Division of Biological Science, Graduate School of Science, Nagoya University, in 2004. In 2010, he completed his doctorate degree in science at Nagoya University, following which he received a GCOE pre-fellowship at Nagoya University. In 2011, he was awarded a research fellowship for young scientists (PD), and he has been in his current position since 2014.
***
“I was interested in female gametophyte, which is important for fertilization,” said Dr. Maruyama, remembering his time as a doctorate student. Through the pre-fellow system in the global COE program at Nagoya University, he joined the Higashiyama laboratory and expanded his interests in the plant fertilization mechanism.
Research is a never-ending process because questions are continuously arising. The plant fertilization story was revealed through such a procedure and I am looking forward to the next chapter (by AU)
Links
- Institute of Transformative Bio-Molecules (WPI-ITbM) HP http://www.itbm.nagoya-u.ac.jp/
- JST ERATO Higashiyama Live Holonics Project HP http://www.liveholonics.com/en/
- Higashiyama Laboratory HP http://www.higashiyama-lab.com/
- Institute for Advanced Research, Nagoya University, HP http://www.iar.nagoya-u.ac.jp/~iar/?lang=en
- Link to this article (from a search-result)
http://www.cell.com/cell/abstract/S0092-8674%2815%2900305-0
Daisuke Maruyama, Ronny Völz, Hidenori Takeuchi, Toshiyuki Mori, Tomoko Igawa, Daisuke Kurihara, Tomokazu Kawashima, Minako Ueda, Masaki Ito, Masaaki Umeda, Shuh-ichi Nishikawa, Rita Groß-Hardt, and Tetsuya Higashiyama.
Rapid Elimination of the Persistent Synergid through a Cell Fusion Mechanism.
Cell. 161: 907 (2015).
(First published on May 7, 2015; doi: 10.1016/j.cell.2015.03.018)
- Link to related article1 (from a search-result)
http://www.nature.com/nature/journal/v458/n7236/full/nature07882.html
Okuda S., Tsutsui H., Shiina K., Sprunck S., Takeuchi H., Yui R., Kasahara R.D., Hamamura Y., Mizukami A., Susaki D., Kawano N., Sakakibara T., Namiki S., Itoh K., Otsuka K., Matsuzaki M., Nozaki H., Kuroiwa T., Nakano A., Kanaoka M.M., Dresselhaus T., Sasaki N., Higashiyama T.
Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells.
Nature, 458: 357 (2009).
(First published on March 19, 2009; doi: 10.1038/nature07882)
- Link to related article2 (from a search-result)
http://www.cell.com/current-biology/abstract/S0960-9822(12)00402-2
Ryushiro D. Kasahara, Daisuke Maruyama, Yuki Hamamura, Takashi Sakakibara, David Twell, and Tetsuya Higashiyama.
Fertilization Recovery after Defective Sperm Cell Release inArabidopsis.
Curr. Biol. 22: 1084 (2012).
(First published on June 19, 2012; doi: 10.1016/j.cub.2012.03.069)
- Link to related article3 (from a search-result)
http://www.cell.com/developmental-cell/abstract/S1534-5807(13)00184-6
Daisuke Maruyama, Yuki Hamamura, Hidenori Takeuchi, Daichi Susaki, Moe Nishimaki, Daisuke Kurihara, Ryushiro D. Kasahara, and Tetsuya Higashiyama.
Independent Control by Each Female Gamete Prevents the Attraction of Multiple Pollen Tubes.
Dev. Cell 25: 317 (2013).
(First published on May 13, 2013; doi: 10.1016/j.devcel.2013.03.013)
NU Research
(English)