Dr. Shohei Saito, an Assistant Professor at the Research Center for Materials Science, Nagoya University, has developed new molecules that change their luminescent color according to environmental conditions and external stimuli, and enabled visualization of state-related variations in materials doped with the molecules. His research, which can be applied to various fields such as material development and infrastructure safety inspections, has attracted wide attention. In 2014, Dr. Saito received the Young Scientists' Prize of the Commendation for Science and Technology by the Minister of Education, Culture, Sports, Science and Technology.
"Material Imaging"―Visualizing Material State Variations ―
Luminescent molecules emit light when releasing energy received from UV light. Molecules can have various structures; some are flexible and easy to transform, whereas others are rigid and cannot change form. In general, rigid molecular structures fit better for luminescent molecules because they have better luminance efficiency. However, because rigid structures are generally not transformable, the luminescent color of the molecule is limited to one color.
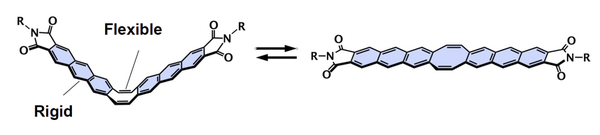
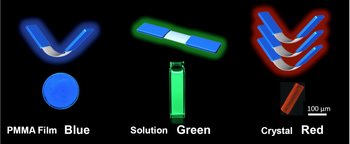 Dr. Saito considered a new approach and designed a luminescent molecule that has a flexible core structure (8-carbon ring) with rigid wing-like structures (6-carbon rings). The flexible core can transform as the environment changes with a corresponding change of luminescent color to blue, green, and red. In a solid, the molecule cannot move, has a V-shaped structure, and emits blue luminescence. In a liquid, where it can move freely, the molecule transforms from a V-shaped to a planar structure and emits green light. In the crystalline form, many V-shaped structures build up layers that generate intermolecular interaction and emit red light. The luminescence can be observed by the naked eye. By doping the newly designed molecule onto a material and observing the changes in colors, we can identify the state variation in the material.
Dr. Saito considered a new approach and designed a luminescent molecule that has a flexible core structure (8-carbon ring) with rigid wing-like structures (6-carbon rings). The flexible core can transform as the environment changes with a corresponding change of luminescent color to blue, green, and red. In a solid, the molecule cannot move, has a V-shaped structure, and emits blue luminescence. In a liquid, where it can move freely, the molecule transforms from a V-shaped to a planar structure and emits green light. In the crystalline form, many V-shaped structures build up layers that generate intermolecular interaction and emit red light. The luminescence can be observed by the naked eye. By doping the newly designed molecule onto a material and observing the changes in colors, we can identify the state variation in the material.
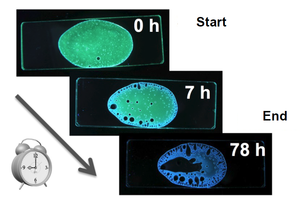 When Dr. Saito added the molecule to an adhesive bond and observed the material under UV light, the part that was dried and hardened produced blue luminescence, whereas the remainder of the material emitted green luminescence. Applying the molecule to various materials allows easy visualization and identification of state variations that could not previously be confirmed. Currently, Dr. Saito's challenge is to apply the molecule to epoxy resin and other industrial adhesives. His research is widely anticipated to contribute to material development.
When Dr. Saito added the molecule to an adhesive bond and observed the material under UV light, the part that was dried and hardened produced blue luminescence, whereas the remainder of the material emitted green luminescence. Applying the molecule to various materials allows easy visualization and identification of state variations that could not previously be confirmed. Currently, Dr. Saito's challenge is to apply the molecule to epoxy resin and other industrial adhesives. His research is widely anticipated to contribute to material development.
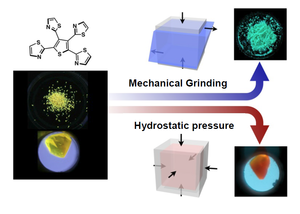
Moreover, Dr. Saito discovered that external stimuli, as well as environmental variations, could influence luminescence color. Previously, many luminescent molecules that change color when in the mechanically ground state have been reported. However, few molecules are known to change luminescence color because of ambient pressure. Dr. Saito developed a new molecule that normally emits yellow light but produces green luminescence in the mechanically ground state and red luminescence under pressure. By observing the color, we can understand what type of force acted on the material. It is expected that this development will be applied to shock sensors and stress sensors.
Why did you become a researcher?
"I did not really intend to enter academia. But when I was a junior high school student, I really enjoyed classes involving experiments. I attended Musashi Junior High School in Tokyo, which has a unique curriculum and is famous for its free school spirit. Under their educational motto, "Inquire and Examine Yourself," independent research was assigned in physics and chemistry classes. The harder I tried, the more my work was evaluated highly, and I enjoyed researching very much. After high school, I entered Kyoto University and was enchanted by chemistry because I could actually touch the materials and design experiments. I chose to work in a chemistry laboratory, and I was influenced a lot by my supervisor, Professor Atsuhiro Osuka and senior students. I went on to do a PhD program because I wanted to research a novel molecule that I had developed when I was a first-year Master's student. Just after the completion of my PhD, I was invited to join Nagoya University by Professor Shigehiro Yamaguchi. At Nagoya University, I enjoy broadening the range of my research through collaboration with researchers from various backgrounds."
Message to Young Students

"I have found that many people do not push themselves when they encounter difficulties in their studies. I believe it is very important to work even harder when it comes to a showdown. If you keep challenging your limit, you will realize that you can push the limit gradually. When you overcome difficulties alone, the experience will enhance your self-confidence and pride. Once you acquire confidence, be conscious that you could be a leading player in future research and think about what you can do for society."
Links
Research Information
Molecular Technology and Creation of New Functions
Researcher's Information
Nagoya University Faculty Member Profile
The Yamaguchi Group, Department of Chemistry, Graduate School of Science, Nagoya University
Research Center for Materials Science, Nagoya University