A catalyst that can be used to produce pharmaceutically relevant molecules is spontaneously assembled from its constituent parts in solution
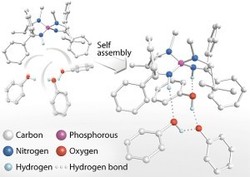
Fig. 1: Self-assembly of a chiral catalyst. The four precursor components (top left) spontaneously form a cyclic structure (right) held together by hydrogen bonds, which acts as a highly effective catalyst for the formation of useful chiral organic molecules.
c 2010 Nagoya University
The vast majority of biological molecules are chiral, which means they exist in only one of two mirror-image forms called enantiomers, and their effects are often modulated by chiral drugs. It is, however, difficult to prepare only one of two chiral forms of a drug molecule because most of the physical properties of the two forms are identical.
One way to produce a single enantiomer is to use a chiral catalyst. This allows a small amount of one chiral molecule in the catalyst to 'direct' the formation of another in the desired product.
This process, called asymmetric catalysis, has rapidly become an important area of synthetic chemistry research, but it is often necessary to resort to the screening of many different catalysts. The catalysts can be relatively complex molecules, so while this may be an effective strategy, it does involve a large investment of resources in their synthesis. Now, writing in the journal Science, Takashi Ooi and co-workers from Nagoya University's Global Center of Excellence for Materials and Molecular Functions describe the spontaneous assembly of an asymmetric catalyst from four simple molecules1.
"Our catalyst is held together by relatively weak hydrogen bonds and ionic interactions rather than the more usual covalent bonds," explains Ooi. "These types of interactions are the same types harnessed by nature in the three-dimensional structures of enzymes and nucleic acids."
The discovery of the catalyst reported by Ooi began with a serendipitous observation. The researchers had prepared a tetraaminophosphonium salt (Fig. 1) as part of a broader study on chiral organic ion pairs. Analysis of crystals of this compound revealed that in the solid state, the tetraaminophosphonium ion was associated with two molecules of phenol and a phenoxide ion.
"We noted that the chirality of the tetraaminophosphonium ion was relayed through the structure by hydrogen bonding interactions, and wondered if we could use this as the basis for a catalyst," says Ooi.
The researchers realized that the phenoxide part of the structure could be replaced by a reactive intermediate called an enolate. They were then able to show that their catalyst induces selectivity in reactions of the enolates to form useful chiral products.
In the immediate future, Ooi and his colleagues plan to investigate what other types of catalyst can be formed in this way. "We can independently vary the organic ion, the bridging molecules and the anion," he says. "This will help us to understand the generality of the system and turn it into a powerful and practical method for catalyst design."
Affiliated Researchers
The Nagoya University affiliated researchers mentioned in this highlight are from the Elucidation and Design of Materials and Molecular Functions GCOE program.
Reference
- Uraguchi, D., Ueki, Y. & Ooi, T. Chiral organic ion pair catalysts assembled through a hydrogen-bonding network. Science 326, 120-123 (2009). | article