A catalytic DNA molecule modified with photoactive units opens the door to photoregulated gene expression
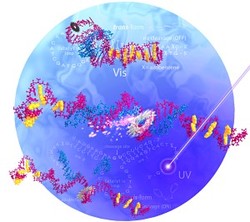
Fig. 1: Schematic illustration showing the light-activated DNA nanomachine cleaving an RNA molecule. Under visible light (top), the azobenzene (yellow)-modified hairpin-shaped DNA molecule (purple) binds to the RNA strand (blue) but remains closed and inactive. Under UV light, it opens (middle) and cleaves the RNA strand (bottom).
c 2010 H. Asanuma, X.G. Liang
Nanotechnology has allowed the construction of many tiny structures with mechanical functions, such as single-molecule propellers and motors. Now, a team led by Hiroyuki Asanuma and Xingguo Liang from the Nagoya University GCOE for Chemistry has designed a DNA-based nanomachine that binds and cleaves single-strand RNA molecules using light1. Many researchers have exploited the ability of single DNA strands to spontaneously hybridize with their complementary strands for the preparation of complex DNA nanomachines. However, such machines often require a constant supply of chemical inputs to function, and these 'molecular fuels' generate by-products that accumulate as waste, restricting their practical use. Asanuma and his team got round this problem by choosing light as a clean energy source to power their DNA nanomachine. At the heart of their new ultra-small device is a hairpin-shaped catalytic DNA molecule called a DNAzyme, which can cleave RNA strands. By modifying the DNAzyme with organic azobenzene compounds -- molecules that can reversibly switch geometric arrangements when exposed to certain wavelengths of light -- the researchers were able to controllably open and close the hairpin catalyst using optical techniques. Asanuma and his team found that the azobenzene units adopted a planar, linear geometry under visible light, keeping the hairpin tightly closed and inactive. Under ultraviolet (UV) light, the same materials changed into non-planar crescent shapes. This UV light-induced change in geometry caused the hairpin to open, activating the DNAzyme (Fig. 1). The team also found that they could repeat this open-close cycle many times by illuminating the nanomachine alternately with UV and visible light, without affecting the catalytic activity. "Planar azobenzene stabilizes the duplex by stacking interactions whereas non-planar azobenzene greatly destabilizes it by steric hindrance, resulting in the reversible and complete photoregulation of DNA," says Asanuma. The researchers observed that their DNA nanomachine was 15-40 times more active under UV light than under visible light, cleaving more strands of RNA. They also tested the catalytic activity towards different RNA sites and discovered that their modified DNAzyme cleaved sites that resisted the original DNAzyme. According to Asanuma, previous attempts at combining azobenzene and DNAzymes were too specific. "Our strategy is versatile enough to be applicable to other DNAzymes because the geometry change is based on reversible hybridization," says Asanuma. The researchers believe that their DNA nanomachine could control gene expression in living cells. What's more, they are currently investigating other light-responsive systems that could store and deliver drugs, proteins, or other molecules to specific targets.
Affiliated Researchers
The Nagoya University affiliated authors mentioned in this highlight are from the Elucidation and Design of Materials and Molecular Functions GCOE program of the Department of Chemistry.
Reference
- Zhou, M., Liang, X., Mochizuki, T. & Asanuma, H. A light-driven DNA nanomachine for the efficient photoswitching of RNA digestion. Angew. Chem. Int. Ed. 49, 2167-2170 (2010). | article