Highlights
Highlights
Kidney disease: A new target
- ツイート
- 2010/09/30
A growth factor expressed after kidney damage could be a key target in the fight against chronic kidney disease
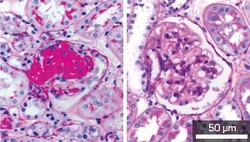
Fig. 1: Stained kidney cells showing the development of severe sclerosis with excess extracellular matrix (red) in glomerular cells from normal mice (left) but not in Mdk-deficient mice (right).
Kidney and cardiovascular diseases are partly caused by over-activity in the renin-angiotensin system (RAS), a hormonal pathway that regulates blood pressure and fluid balance. For this reason, drugs that inhibit the RAS are the first choice of treatment for chronic kidney disease. However, the underlying molecular mechanisms that control the RAS are poorly understood.
Kenji Kadomatsu from the Nagoya University GCOE for Molecular Medicine and colleagues1 have now identified a growth factor that acts as a key regulator of the RAS. The growth factor, called midkine, mediates cross-talk between the kidney and lungs in chronic kidney disease.
Kadomatsu and his co-workers used genetic engineering to create mutant mice lacking the midkine gene (Mdk). These animals developed normally and had blood pressure comparable to that of normal mice.
However, when the researchers destroyed parts of the mouse kidneys, they saw some striking differences. The blood pressure of the wild-type mice increased markedly two weeks after surgery, but the mutants were unaffected. The wild-type animals also exhibited progressive kidney failure, but the mutants did not.
The researchers found that the hypertension and kidney damage in the wild-type animals was caused by elevated levels of angiotensin II, a protein that causes blood vessels to constrict. Both symptoms could be reduced by administering temocapril, a drug that blocks angiotensin II formation by inhibiting the angiotensin-converting enzyme (ACE).
Further investigation revealed that the destruction of the kidneys led to a significant increase in ACE expression in the lungs of the normal mice, but not those lacking the Mdk gene. This suggests that midkine normally acts to induce ACE expression following kidney damage.
Finally, the researchers investigated how midkine expression in the lungs is induced following kidney damage. In the lungs of the normal mice, but not the mutants, they found significant increases in the expression levels of Nox-1, Nox-2 and Nox-4. These genes encode enzymes that initiate the production of highly reactive oxygen atoms, which can damage cells by a process called oxidative stress.
Interestingly, Kadomatsu's group has previously shown that oxidative stress induces midkine expression. Their new study provides insights into chronic kidney disease by showing that kidney damage induces oxidative stress in the lungs.
"Drugs targeting midkine may relieve not only hypertension but also inflammation," says Kadomatsu. "Chronic inflammatory diseases such as chronic kidney disease, pulmonary fibrosis and chronic heart disease are serious problems in developed countries, so midkine could be an intriguing target."
Kidney and cardiovascular diseases are partly caused by over-activity in the renin-angiotensin system (RAS), a hormonal pathway that regulates blood pressure and fluid balance. For this reason, drugs that inhibit the RAS are the first choice of treatment for chronic kidney disease. However, the underlying molecular mechanisms that control the RAS are poorly understood.
Kenji Kadomatsu from the Nagoya University GCOE for Molecular Medicine and colleagues1 have now identified a growth factor that acts as a key regulator of the RAS. The growth factor, called midkine, mediates cross-talk between the kidney and lungs in chronic kidney disease.
Kadomatsu and his co-workers used genetic engineering to create mutant mice lacking the midkine gene (Mdk). These animals developed normally and had blood pressure comparable to that of normal mice.
However, when the researchers destroyed parts of the mouse kidneys, they saw some striking differences. The blood pressure of the wild-type mice increased markedly two weeks after surgery, but the mutants were unaffected. The wild-type animals also exhibited progressive kidney failure, but the mutants did not.
The researchers found that the hypertension and kidney damage in the wild-type animals was caused by elevated levels of angiotensin II, a protein that causes blood vessels to constrict. Both symptoms could be reduced by administering temocapril, a drug that blocks angiotensin II formation by inhibiting the angiotensin-converting enzyme (ACE).
Further investigation revealed that the destruction of the kidneys led to a significant increase in ACE expression in the lungs of the normal mice, but not those lacking the Mdk gene. This suggests that midkine normally acts to induce ACE expression following kidney damage.
Finally, the researchers investigated how midkine expression in the lungs is induced following kidney damage. In the lungs of the normal mice, but not the mutants, they found significant increases in the expression levels of Nox-1, Nox-2 and Nox-4. These genes encode enzymes that initiate the production of highly reactive oxygen atoms, which can damage cells by a process called oxidative stress.
Interestingly, Kadomatsu's group has previously shown that oxidative stress induces midkine expression. Their new study provides insights into chronic kidney disease by showing that kidney damage induces oxidative stress in the lungs.
"Drugs targeting midkine may relieve not only hypertension but also inflammation," says Kadomatsu. "Chronic inflammatory diseases such as chronic kidney disease, pulmonary fibrosis and chronic heart disease are serious problems in developed countries, so midkine could be an intriguing target."
Affiliated Researchers
The Nagoya University affiliated authors mentioned in this highlight are from the Integrated Functional Molecular Medicine for Neuronal and Neoplastic Disorders GCOE program of the Graduate School of Medicine.
Reference
- Hobo, A., Yuzawa, Y., Kosugi, T., Kato, N., Asai, N., Sato, W. Maruyama, S., Ito, Y., Kobori, H., Ikematsu, S. et al. The growth factor midkine regulates the renin-angiotensin system in mice. Journal of Clinical Investigation 119, 1616-1625 (2010). | article
NU Research
(English)

