Professor Cathleen Crudden and Designated Assistant Professor Masakazu Nambo at the Institute of Transformative Bio-Molecules, Nagoya University, successfully developed a novel cross-coupling reaction that enables the efficient synthesis of triarylmethanes. Their paper was published online in Angewandte Chemie International Edition on December 5, 2013.
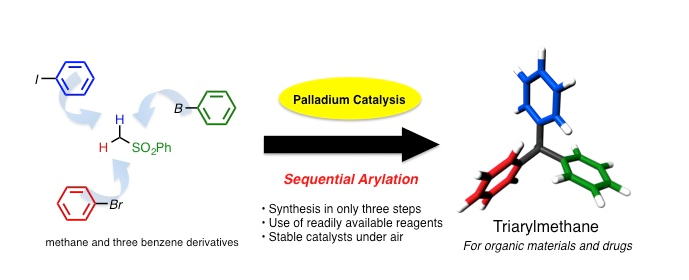
Triarylmethanes are essential in our lives and are used in dyes, fluorescent probes, and medicines. Triarylmethanes comprise one carbon atom and three benzene rings. The synthesis of triarylmethanes is very difficult, because undesired positional isomers are often generated, which contribute to lower yields and separation/purification problems. Some cross-coupling reactions have been reported using nickel and palladium catalysts, which overcome these issues; however, these methods have problems such as the instability of the catalysts and excessive number of steps required to complete the synthesis.
Thus, to solve the problem of triarylmethane synthesis, Prof. Crudden and Prof. Nambo focused on simplifying the synthetic process. They combined three different palladium catalysts, which enabled the sequential introduction of benzene rings to a methane derivative. As a result, they succeeded in synthesizing triarylmethanes in only three steps. Moreover, by using this newly developed coupling reaction, they succeeded in synthesizing an anti-breast cancer compound in very few steps. This reaction has been attracting global attention because the necessary raw materials and catalysts for the reaction are easily available and stable under atmospheric conditions. Furthermore, by this method, triarylmethane derivatives can be prepared in very few steps. Thus, this new reaction will accelerate research in systems biology at the Institute of Transformative Bio-Molecules.
Designated Assistant Professor Masakazu Nambo
Prof. Nambo chose to study organic chemistry because he was fascinated by cross-coupling reactions in undergraduate school. Cross-coupling reactions are innovative methods in synthetic chemistry that enable chemical conversions that cannot be accomplished using conventional organic chemistry. To simplify and streamline chemical syntheses, Prof. Nambo believes that there are possibly many cross-coupling reactions that need to be developed. Currently, he is an investigator at the Institute of Transformative Bio-Molecules, Nagoya University1 and develops new reactions that can be used in various disciplines.
1.The World Premier International Research Center Initiative (WPI Program)was launched by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), and aims to build research centers with world-leading researchers.
Outlook for the Future
"I believe that this method for the preparation of triarylmethanes will contribute to the discovery of new molecular groups with new functionality such as biological activity. I would like to develop a coupling method, which is plain and simple, and can be practically used by researchers in different fields as well as by those in chemistry."
Message to Young Students
 "Only one discovery can make a significant change to the world. I am constantly motivated and thrilled by the discoveries I make during my research. Moreover, there is plenty of opportunity to solve challenging problems. Why not accept the challenge and experience the excitement of research?"
"Only one discovery can make a significant change to the world. I am constantly motivated and thrilled by the discoveries I make during my research. Moreover, there is plenty of opportunity to solve challenging problems. Why not accept the challenge and experience the excitement of research?"
Links
Research Information
Designated Assistant Professor Masakazu Nambo Information