Highlights
Highlights
The development of novel "stimuli-sensitive" hydrogels for various applications
- Read in Japanese
- ツイート
- 2015/02/04
- Graduate School of Engineering
- Project Assistant Prof. Abu Bin Imran
- Associate Prof. Yukikazu Takeoka
PDF version is available here:
nureseach_e002.pdf
Associate Prof. Yukikazu Takeoka and his research group at the Graduate School of Engineering, Nagoya University invented extremely stretchable, highly tough and strong hydrogels using novel, freely movable polyrotaxane cross-linkers. In addition, the response speed of the stimuli-sensitive hydrogels prepared using the cross-linkers is dramatically enhanced when compared with that of conventional polymer gels.
Conventional polymer gels typically exhibit slow responses and are mechanically brittle. Consequently, it has been difficult to apply them in practical applications using changes in volume and surface area that occur in conventional polymer gels. With their improved properties, the new stimuli-sensitive hydrogels should be suitable for various practical applications, including artificial muscles, drug delivery systems, sensors, and culture beds for regenerative medicine.
This research was published in the international academic journal "Nature Communications (electronic version)" in the United Kingdom on October 8, 2014. → Link to Nagoya University Press Release
―Extending, bending, twisting, and pressing― With changing volumes and shapes in response to various stimuli, the super polymer gels were fabricated here at Nagoya University.
Soft contact lenses, diapers, photo films, gummies, tofu, etc.
All of these things are made of "gels", and they all are part of our daily lives.
"There are many gel-like foods in the East Asia."
As food is viewed as the symbiosis of culture and science, not surprisingly, Japan is a world leader in gel research. Associate Prof. Yukikazu Takeoka and his research group at the Graduate School of Engineering, Nagoya University are recognized as top researchers in the field of polymer gels.
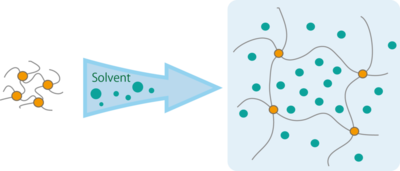
Polymer gels are cross-linked polymer chains that form a three-dimensional network structure. These gels swell when immersed into a solvent due to absorption and retention of the solvent in the gaps within the network (Figure 1). Because of their unique properties, polymer gels can function as molecular sieves and absorbers for liquid and even vibrations. They are also very easy to prepare and consequently have found practical use in multiple fields.
Figure 1. The schematic diagram of the network structure of gel, swelling when immersed into a solvent.
However, even though polymer gels have great potential, they can suffer from brittleness. Until this "fragility" can be overcome, their use in more advanced applications, such as artificial muscles, drug delivery systems, sensors, and culture beds for regenerative medicine, will remain very limited.
Associate Prof. Takeoka and Project Assistant Prof. Abu Bin Imran took the first step toward the practical use of polymer gels with the preparation of newly developed cross-linking points that move freely, resulting in hydrogels with high stimuli-sensitive characteristics (extensibility and tenacity). Importantly, the preparation of these advanced gels involves a simple process similar to the one used for the synthesis of conventional polymer gels.
*******
"I was originally studying biomaterials."
In his student days, Associate Prof. Takeoka was committed to the development of polymer gels for use in drug delivery systems in the field of medical engineering. By using a gel that "releases medicine only when it is heated," the delivery of a drug substance on the surface of the gel could be controlled, resulting in its release only in the areas where the drug is needed, thereby suppressing any side effects.
Therefore, to control the drug distribution in the body, polymer gels that are stimuli-sensitive and flexible are required.
To date, researchers have developed polymer gels based on exfoliated clay as cross-linkers, and also gels consisting of double network, primary networks of hard polymers and soft secondary networks. However, in the former case, because the cross-linking sites that establish the networks are connected by hydrogen bonds, these gels gradually dissolve in water. In the latter case, the gels often degrade after repeated use. Thus, even after approximately 30 years of research, there are few examples for the practical application of stimuli-sensitive hydrogels.
"I was asked to study this problem because I had been successful at introducing light responsive functionality to gels."
Associate Prof. Takeoka also has an interest in the structural color of materials or the color resulting from the physical construction of a substance. Applying this interest to polymer gels, he was able to develop sensors based on gels that change color due to the change in the volume of the gel (Bragg reflection) in response to various environmental stimuli. With expertise in both soft materials and structural color, Associate Prof. Takeoka began working with Prof. Takahiro Seki at the Graduate School of Engineering at Nagoya University and conducting joint research with Prof. Kohzo Ito at the Graduate School of Frontier Sciences, The University of Tokyo.
Prof. Ito and his research group invented polymer gels with freely movable cross-linking points (Okumura and Ito, Adv. Mater. (2001) 13: 485-7) and advanced the field of soft and strong polymer materials. This technology was patented and Advanced Softmaterials Inc. (http://www.asmi.jp/en/) was founded. The company offers a slide ring material (Figure. 2) that exhibits a pulley effect.
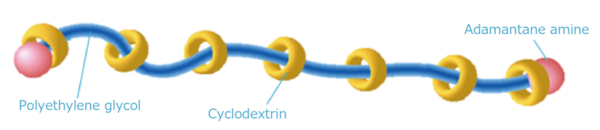 Figure 2. A slide ring material. (Figure: by courtesy of Advanced Softmatrials Inc.)
Figure 2. A slide ring material. (Figure: by courtesy of Advanced Softmatrials Inc.)
The slide ring material is a three-dimensional polymer network with a polyrotaxane (PR) structure (Harada et al. Nature (1992) 356: 325-7) consisting of three molecular (supramolecular) complexes, a linear polymer polyethylene glycol (PEG), the cyclic molecule cyclodextrin (CD), and adamantane amine, a stopper molecule that ends the polymer chain. Under certain conditions, the pulley effect, in which the CD can move freely on the PEG, can be expected.
"Using this PR as a cross-linker, we believed that stimuli-sensitive hydrogels could be prepared by installing the pulley effect in the polymer network."
Associate Prof. Takeoka and Project Assistant Prof. Imran thus began developing stimuli-sensitive hydrogels with flexible mechanical properties using a PR slide ring material.
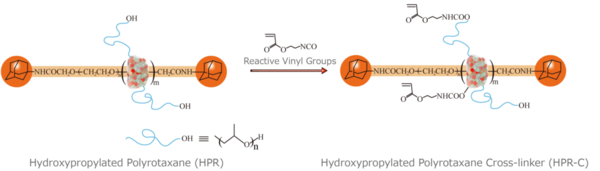
Project Assistant Prof. Imran first modified the hydrophilic PR, HPR with reactive vinyl groups in order to synthesize a cross-linker (HPR-C) (Figure 3). He then prepared a mixed solution of this cross-linker and N-isopropylacrylamide (NIPA), a known thermosensitive monomer, in water. Ammonium persulfate (APS) was used as the initiator of the gelation reaction, and tetramethylethylenediamine (TEMED) served as a reaction accelerator.
Figure 3. A synthesis of a cross-linker, HPR-C. (Figure was cited from : Abu Bin Imran et al. Nature Communications (2014) 5: 5124 Copyright © 2014, Rights Managed by Nature Publishing Group.)
However, the resultant NIPA-HPR-C hydrogel, despite the use of a PR cross-linker, was still "fragile", and the pulley effect was not observed.
"I was disappointed but had no intention to give up."
Project Assistant Prof. Imran next attempted to examine the volume changes of hydrogels with the movable cross-linking points in response to changes in the environment. In stimuli-sensitive gels, when a change in the surrounding environment occurs, such as a change in the temperature or solvent composition, the polymer chains constituting the network shrink or expand accordingly, resulting in a change in the volume of the polymer gel. In general, when exposed to materials with ionic groups, such gels absorb the solvent and become swollen.
To study the effect of an ionic material on the NIPA-HPR-C hydrogel, Project Assistant Prof. Imran added a small amount of sodium acrylate (AAcNa) to the gel (Figure. 4).
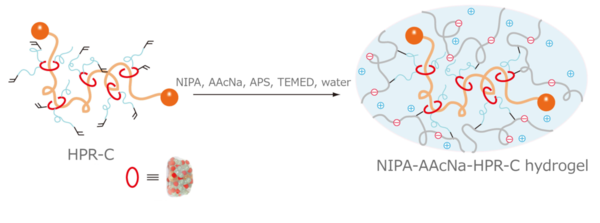 Figure 4. A synthesis of hydrogel by using HPR-C. (Figure was cited from : Abu Bin Imran et al. Nature Communications (2014) 5: 5124 Copyright © 2014, Rights Managed by Nature Publishing Group.)
Figure 4. A synthesis of hydrogel by using HPR-C. (Figure was cited from : Abu Bin Imran et al. Nature Communications (2014) 5: 5124 Copyright © 2014, Rights Managed by Nature Publishing Group.)
The resultant hydrogel was very flexible and could expand to well over 10 times the size possible for conventional polymer gels. In addition, it was not easy to cut, even using a sharp cutter (Figure. 5).
Figure 5. The resultant hydrogel in response to various environmental stimuli (extending, pressing, twisting, knotting, and cutting by a sharp cutter). (Figure was cited from : Abu Bin Imran et al. Nature Communications (2014) 5: 5124 Copyright © 2014, Rights Managed by Nature Publishing Group.)
By introducing ionic groups into the polymer network, the entire polymer network exhibited an increased ability to generate osmotic pressure due to the interactions of the ions in the ionic group with the polymer network inside the hydrogel. In addition, it is believed that the cross-linking sites were freely movable because the HPR-C used as the cross-linker became significantly extended in the mesh of the network (Figure.6).
 Figure 6. The swallen hydrogel. (Figure was cited from : Abu Bin Imran et al. Nature Communications (2014) 5: 5124 Copyright © 2014, Rights Managed by Nature Publishing Group.)
Figure 6. The swallen hydrogel. (Figure was cited from : Abu Bin Imran et al. Nature Communications (2014) 5: 5124 Copyright © 2014, Rights Managed by Nature Publishing Group.)
Furthermore, Project Assistant Prof. Imran proposed that insertion of the ionic groups directly into the PR, rather than extending to the stimulus responsive portions of the polymer network, would be sufficient for achieving similar behavior. As a result, without changing the stimuli-sensitive properties of the polymer, it could be possible to exert the pulley effect in the hydrogel.
First, he prepared an ionic PR and then as before synthesized a cross-linker modified with vinyl groups (iPR-C) (Figure. 7).
Figure 7. A synthesis of an ionic coss-linker, iPR-C. (Figure was cited from : Abu Bin Imran et al. Nature Communications (2014) 5: 5124 Copyright © 2014, Rights Managed by Nature Publishing Group.)
Second, he produced a hydrogel using only NIPA and the iPR-C (NIPA-iPR-C) without using AAcNa. Because the charge in the polymer gel was concentrated only around the cross-linker (Figure. 8), the pulley effect was successfully applied to the hydrogel without affecting the stimuli-sensitive properties of the polymer.
 Figure 8. The state of charge in the hydrogel produced by NIPA and iPR-C. (Figure was cited from : Abu Bin Imran et al. Nature Communications (2014) 5: 5124 Copyright © 2014, Rights Managed by Nature Publishing Group.)
Figure 8. The state of charge in the hydrogel produced by NIPA and iPR-C. (Figure was cited from : Abu Bin Imran et al. Nature Communications (2014) 5: 5124 Copyright © 2014, Rights Managed by Nature Publishing Group.)
You can see here in the movie the "stimuli-sensitive" hydrogel ―you may doubt it is a gel, or rather a rubber:
A Hydrogel NOT exhibiting the pully effect (Movie was cited from : Abu Bin Imran et al. Nature Communications (2014) 5: 5124 Copyright © 2014, Rights Managed by Nature Publishing Group.)
A Hydrogel using a movable cross-linker, HPR-C (Movie was cited from : Abu Bin Imran et al. Nature Communications (2014) 5: 5124 Copyright © 2014, Rights Managed by Nature Publishing Group.)
A Hydrogel using a movable cross-linker, iPR-C (Movie was cited from : Abu Bin Imran et al. Nature Communications (2014) 5: 5124 Copyright © 2014, Rights Managed by Nature Publishing Group.)
*******
"It could be manufactured even by a young child."
One of the important features of this new hydrogel technology is that the polymer gels can be produced using a simple process that can be performed by anyone.
The development of stimuli-sensitive hydrogels that undergo changes in volume more rapidly and by a greater percentage than conventional polymer gels in response to a change in the surrounding environmental or certain types of stimulation should lead to expanded applications for these advanced materials.
Entirely new "stimuli-sensitive" hydrogels are now ready for use in a wide variety of applications
―as a result of this inspired advancement in science and technology.
(Ayako Umemura)
Researchers featured in this article
Dr. Imran(left)and Dr. Takeoka(right)
Dr. Yukikazu Takeoka【Associate Professor, Graduate School of Engineering, Nagoya University】
 Yukikazu Takeoka obtained his doctorate degree in engineering from Sophia University. In 1996, he worked at the Department of Physics, Massachusetts Institute of Technology as a postdoctoral fellow under a grant from the Uehara Memorial Foundation. In 1998, Dr. Takeoka moved to the College of Engineering, Yokohama National University as an assistant and then, in 2004, became an associate professor in the Graduate School of Engineering at Nagoya University, the position he holds today.
Yukikazu Takeoka obtained his doctorate degree in engineering from Sophia University. In 1996, he worked at the Department of Physics, Massachusetts Institute of Technology as a postdoctoral fellow under a grant from the Uehara Memorial Foundation. In 1998, Dr. Takeoka moved to the College of Engineering, Yokohama National University as an assistant and then, in 2004, became an associate professor in the Graduate School of Engineering at Nagoya University, the position he holds today.
Associate Prof. Takeoka has received many awards during his career:
The 1995 JORGE HELLER Journal of Controlled Release/CRS Outstanding Paper Award (1995);
Award for Encouragement of Research in Materials Science; The Materials Research Society of Japan (2000);
Award for Encouragement of Research in Materials Science; The Materials Research Society of Japan (2001);
Tejima Memorial Award; Tejima Commemorative Foundation (2001);
Konica Minolta Award 2007 for Encouragement of Image Science; Konica Minolta Imaging Science Foundation (2007);
Award for Encouragement of Research in The Kao Foundation for Arts and Sciences (2009);
Academic Prize of the Nagai Foundation for Science and Technology (2013).
***
Dr. Takeoka supervises his students and researchers, while he strives for his own studies "to cherish the intuition." He seems to greatly enjoy getting closer to the young researchers to understand what they are looking for.
Dr. Takeoka steadily advances the research field and influences many people. I expect that he will continue to make progress in many interesting areas of research. I would also like to introduce his research on structural colors discussed on NU Research (by AU)
Dr. Abu Bin Imran【Project Assistant Professor, Graduate School of Engineering, Nagoya University】
 Abu Bin Imran is an assistant professor in the Department of Chemistry, Bangladesh University of Engineering and Technology (BUET), Dhaka-1000. He was born in Comilla, Bangladesh in 1980. He received his Bachelor of Science and Master of Science degrees from the Department of Chemistry, Shahjalal University of Science and Technology, Sylhet, Bangladesh, in 2004 and 2006, respectively. Dr. Imran received his PhD degree in 2009 from the Department of Molecular Design and Engineering at Nagoya University, Nagoya, Japan under the supervision of Professor Yukikazu Takeoka. He subsequently worked as a post-doctoral fellow at the New Energy and Industrial Technology Development Organization (NEDO) project, Nagoya University, Nagoya, Japan until October, 2011. Since 2014, Dr. Imran has concurrently served as a project assistant professor at Nagoya University.
Abu Bin Imran is an assistant professor in the Department of Chemistry, Bangladesh University of Engineering and Technology (BUET), Dhaka-1000. He was born in Comilla, Bangladesh in 1980. He received his Bachelor of Science and Master of Science degrees from the Department of Chemistry, Shahjalal University of Science and Technology, Sylhet, Bangladesh, in 2004 and 2006, respectively. Dr. Imran received his PhD degree in 2009 from the Department of Molecular Design and Engineering at Nagoya University, Nagoya, Japan under the supervision of Professor Yukikazu Takeoka. He subsequently worked as a post-doctoral fellow at the New Energy and Industrial Technology Development Organization (NEDO) project, Nagoya University, Nagoya, Japan until October, 2011. Since 2014, Dr. Imran has concurrently served as a project assistant professor at Nagoya University.
Project Assistant Prof. Imran received an award from the Research Center for Materials Backcasting Technology (MBT) in 2010.
***
Dr. Imran smilingly says that "Japan is my second home". During his vacations, he travels around Japan taking photographs and enjoys staying in Japan.
Dr. Imran keeps smiling all the time and says that "I want to study as much as possible here in Japan, because the laboratories in Bangladesh are not as substantial." I believe that he will pursue his research as tenaciously and energetically as his polymer gels. I am looking forward to his further success in the future (by AU)
Links
- Seki laboratory HP http://www.apchem.nagoya-u.ac.jp/06-BS-2/sekilabo/index-j.html
- Graduate School of Engineering HP http://www.engg.nagoya-u.ac.jp/en/index.html
- Advanced Softmaterials Inc. HP http://www.asmi.jp/en/
- Link to this article (from a search-result)
http://www.nature.com/ncomms/2014/141008/ncomms6124/full/ncomms6124.html
Abu Bin Imran, Kenta Esaki, Hiroaki Gotoh, Takahiro Seki, Kohzo Ito, Yasuhiro Sakai and Yukikazu Takeoka
Extremely stretchable thermosensitive hydrogels by introducing slide-ring polyrotaxane cross-linkers and ionic groups into the polymer network.
Nature Communications 5: 5124(2014).
(First published on October 8, 2014; doi:10.1038/ncomms6124)
- Link to related article1 (from a search-result)
http://onlinelibrary.wiley.com/doi/10.1002/1521-4095%28200104%2913:7%3C485::AID-ADMA485%3E3.0.CO;2-T/abstract
Y. Okumura and K. Ito
The polyrotaxane gel: A topological gel by figure-of-eight cross-links.
Advanced Materials 13: 485(2001).
(First published on April 6, 2001; doi:10.1002/1521-4095(200104)13:7<485::AID-ADMA485>3.0.CO;2-T)
- Link to related article2 (from a search-result)
http://www.nature.com/nature/journal/v356/n6367/abs/356325a0.html
AKIRA HARADA, JUN LI & MIKIHARU KAMACHI
The molecular necklace: a rotaxane containing many threaded α-cyclodextrins.
Nature 356: 325(1992).
(Published on March 26, 1992; doi: 10.1038/356325a0)
NU Research
(English)