Highlights
Highlights
A new simple and green synthetic method for the production of pharmaceuticals
- Read in Japanese
- ツイート
- 2016/03/25
- Graduate School of Engineering
- Associate Prof. Manabu Hatano
- Prof. Kazuaki Ishihara
Prof. Kazuaki Ishihara, Associate Prof. Manabu Hatano, and Mr. Katsuya Yamakawa (a graduate student), Graduate School of Engineering at Nagoya University, successfully developed an efficient enantioselective synthetic method of cyanohydrins suitable for the development and production of pharmaceuticals and agricultural chemicals.
The reaction time required for the catalytic enantioselective cyanosilylation of ketones in all the 25 substrates examined in this study ranged from 9 hours to as short as 2 hours, which ranks as the shortest found in the world yet. In addition, high yields were obtained even on an industrial scale; thus, this technique is expected to be of great utility in the development and production of pharmaceuticals and agricultural chemicals in the marketplace.
This research achievement was published online (Angew. Chem. Int. Ed. 2016, 55(12), 4021-4025, DOI: 10.1002/anie.201510682) in Angewandte Chemie International Edition on February 2, 2016, and was selected as the Cover Picture of the journal. → Nagoya University Press Release
A simple and green synthetic method has been newly established. With its short reaction time and high yields of enantioselective products, even on a large scale, the next step is to apply for an academia–industry collaboration.
"Research and development are becoming more advanced, complicated, and large scaled."
Prof. Kazuaki Ishihara, Associate Prof. Manabu Hatano, and their research group at the Graduate School of Engineering at Nagoya University develop and suggest new synthetic methods for the efficient production of complicated chemicals, such as pharmaceuticals and agricultural chemicals. A key to control the synthesis is a catalyst that should be designed for selectivity and be environmentally friendly.
Since catalytic asymmetric synthesis was first developed by Prof. Ryoji Noyori from Nagoya University [a Nobel laureate in chemistry (2001)], the importance and future of catalysts have been focused. An infinite number and variety of chemicals can be synthesized with only a small amount of catalyst; therefore, there is practical certainty as to use catalysts.
In this study, by successfully designing a new catalyst, the research group suggested a unique and efficient enantioselective synthetic method for the production of cyanohydrins suitable for the development and production of pharmaceuticals and agricultural chemicals. The technique can be expected to find further practical use in the preparation of new chemical intermediates to meet the market demand in the future. Moreover, by expanding the synthetic library with the data gathered throughout the work, the insights gained through the development of the catalysts and its synthetic applications will further the advancement of synthetic techniques.
*******
—To handle chemical reactions freely
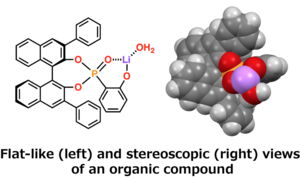 “In general, organic compounds form complicated three dimensions.”
“In general, organic compounds form complicated three dimensions.”
Organic compounds are not flat two dimensional structures as seen on a piece of paper; but in reality, they have stereoscopic or three dimensional structures. That is, even if the structural formula is the same, different spatial alignments of the atoms are distinguished as stereoisomers, which may cause the organic compounds to have different properties.
For example, thalidomide is one of well-known enantiomeric compounds, which have the same relationship as one’s left and right hands: one hand functions as a medicine, whereas the other hand can be poisonous (Figure 1). Here, for mixtures of final products that differ by this chirality, it is necessary to separate the mixed compounds in order to obtain only the one having desired function. It is far better to make only the desired compound in the first place using a selective synthesis.
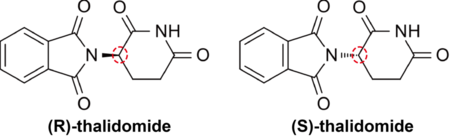 Figure 1. Thalidomide is an enantiomeric compound: R-form functions as a medicine, whereas S-form can be poisonous. Just like a pair of one's right and left hands, its mirror image can be structured with a chiral center (shown in red circle).
Figure 1. Thalidomide is an enantiomeric compound: R-form functions as a medicine, whereas S-form can be poisonous. Just like a pair of one's right and left hands, its mirror image can be structured with a chiral center (shown in red circle).
“In order to handle chemical reactions freely, catalysts are absolutely imperative.”
Prof. Ishihara and his research group worked on the development of catalysts for enantioselective synthetic methods. In this study, they targeted cyanohydrins, which are especially important intermediates in developing and producing pharmaceuticals and agricultural chemicals.
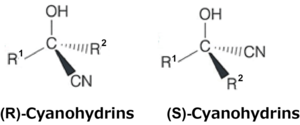
For establishing the method of asymmetric catalytic technology, cyanohydrins having a chiral carbon comprise a variety of highly valued compounds because of their steric structure. In addition, having these composite elements available for the new design of pharmaceuticals and agricultural chemicals stored in the “library of chiral composite elements” can be expected to assist in the further development of asymmetric catalysts or in the production of new chemicals.
—Manufacturing unknown composite elements
Cyanohydrins are compounds with functional moieties, a hydroxy group (-OH) and a cyano group (-CN). For example, the functional compounds can be converted chemically to key synthetic intermediates for pharmaceuticals, such as α-hydroxycarboxylic acids, α-amino acids, and β-hydroxy amines (Figure 2).
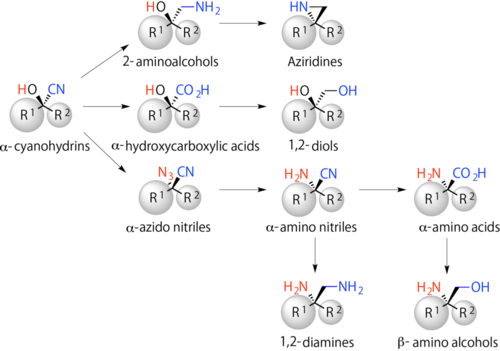 Figure 2. α-cyanohydrins and key synthetic intermediates for pharmaceuticals
Figure 2. α-cyanohydrins and key synthetic intermediates for pharmaceuticals
So far, a number of excellent synthetic methods for the enantioselective cyanosilylation of aldehydes have been reported and already utilized even on an industrial scale. However, to succeed in achieving further structural diversity, cyanohydrins should be synthesized from ketones as well as from aldehydes (figure 3).
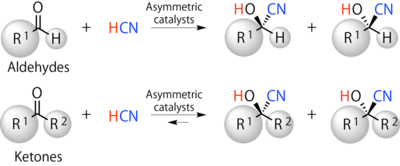 Figure 3. Enantioselective cyanosilylation of aldehydes or ketones. With substituent groups R1 and R2 of Ketones (cf. only R1 of aldehydes), further structural diversity can be achieved. Here, a key is to design aymmetric catalysts.
Figure 3. Enantioselective cyanosilylation of aldehydes or ketones. With substituent groups R1 and R2 of Ketones (cf. only R1 of aldehydes), further structural diversity can be achieved. Here, a key is to design aymmetric catalysts.
This creates some difficulties for the synthetic chemicals.
Ketones, rarely used in asymmetric induction, are less reactive than aldehydes. Scaling-up the production is very difficult because the final products of cyanosilylation of ketones are easily degraded. Researchers therefore have to cope with these limitations.
“Thus, designing a proper catalyst is a key to control the enantioselective reaction.”
Prof. Ishihara explained that potential composite elements that are yet unknown will be produced during this development to enable even better asymmetric catalysts.
—Pursuing an original and simple design of catalysts
“We have been developing the design of catalysts for these dozen years or so.”
Associate Prof. Hatano has been developing original catalysts with his students since Ishihara laboratory was founded. This work has achieved the third generation of catalysts for cyanosilylation.
 Figure 4. The first generation of catalyst. The lithium cation functions as an acid in the catalyst to activate an aldehyde; the phenoxy anion simultaneously serves as a base to activate a cyano reagent.
Figure 4. The first generation of catalyst. The lithium cation functions as an acid in the catalyst to activate an aldehyde; the phenoxy anion simultaneously serves as a base to activate a cyano reagent.
The first generation of catalyst (Figure 4) was developed for the cyanosilylation of aldehydes (Hatano et al. J. Am. Chem. Soc. 2005, 127, 10776).
The production procedure of catalysts is usually cumbersome. However, Associate Prof. Hatano and his research group suggested a rather simple procedure that enabled the commercial production of enantiomeric 1,1’-binaphthol (BINOL) with a lithium source and water. According to the JACS access ranking, the research article had the highest impact to researchers all over the world.
“The interesting thing is Mr. Ikeno, a senior student, performed better in the experiment than me.”
Associate Prof. Hatano noted the importance of the experiment.
In general, the production procedure of catalysts requires avoiding water. At that time, it was the rainy season in Japan; therefore, Associate Prof. Hatano certainly dried out the container, because if it is not perfectly done, even a little water will cause unexpected results.
However, in this experiment, the unexpected result was that a little water was advantageous. Associate Prof. Hatano and Mr. Ikeno worked together and showed that a product was obtained with 58%ee (enantiomeric excess) in the absence of water, whereas, in the presence of water, a product was obtained with 95%ee. Noting the effect of water on this synthesis was of great scientific importance.
 Figure 5. The second generation of catalyst. A lithium source and phenols (Ph) were added in the phosphoric acid catalyst.
Figure 5. The second generation of catalyst. A lithium source and phenols (Ph) were added in the phosphoric acid catalyst.
For the second generation of catalyst (Figure 5), the research group started the cyanosilylation of ketones (Hatano, et al. Adv. Synth. Catal. 2008, 350, 1776).
They decided to use a phosphoric acid catalyst with the BINOL framework. The phosphoric acid catalyst is enantiomeric, and nowadays, it is often used for the conversion reaction because of its practical reaction speed and high selectivity.
Although the phosphoric acid catalyst did not work well initially for this synthesis, Associate Prof. Hatano conceived adding a lithium source in this phosphoric acid catalyst, as in the first generation of catalytic structures.
Acids and bases in catalysts are usually mild to activate substrates; however, if it works as an acid/base cooperative catalyst, the reaction will synergistically be active because of the dual activation. In particular, in the science of this developed catalyst, the lithium cation functions as an acid in the catalyst to activate a ketone; the phenoxy anion simultaneously serves as a base to activate a cyano reagent.
As expected, they succeeded in achieving the enantioselective cyanosilylation of ketones. However, only a limited number of ketones were reactive and the yield was not high, being only the 80% to 89% range.
 Figure 6. The third generation of catalyst. Adding another phenol in the second generation of catalyst, the catalytic activity was increased.
Figure 6. The third generation of catalyst. Adding another phenol in the second generation of catalyst, the catalytic activity was increased.
“The important thing here is to increase the catalytic activity by adding a phenol somewhere inside the catalyst.”
Associate Prof. Hatano revealed that phenols are the key to increase the catalytic activity, and the third generation of catalyst (Figure 6), lithium(I) phosphoryl phenoxide, was designed by adding a phenol to the second generation catalyst.
The research group also devised the cyano reagent to be highly active.
Initially, they used a trimethylsilyl cyanide reagent; however, the reactivity was not strong enough. Next, they tried lithium(I) dicyanotrimethylsilicate(IV) instead. Although it has been considered unsuitable for achieving stereochemical control by asymmetric catalysts, the researchers thought that it might work effectively if combined with the low reactivity of ketones because of its usually high reactivity.
First, they reacted appropriate quantities of water and nBuLi as the same lithium(I) source to prepare lithium(I) silicates(IV) in situ. Then, the lithium(I) silicates(IV) were combined with trimethylsilyl cyanide reagent to produce a lithium(I) dicyanotrimethylsilicate(IV) (Figure 7).
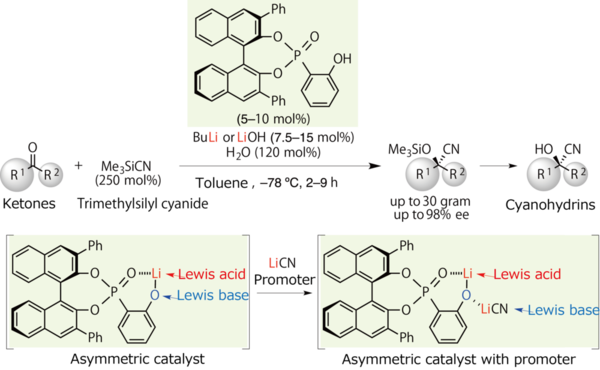 Figure 7. After the reaction of the asymmetric catalyst with the appropriate quantities of water and nBuLi, also combined with a promoter LiCN, a further powerful catalyst was produced.
Figure 7. After the reaction of the asymmetric catalyst with the appropriate quantities of water and nBuLi, also combined with a promoter LiCN, a further powerful catalyst was produced.
“In this synthetic procedure for cyanohydrins, the promoter also plays an important role.”
As they expected, the acid/base cooperative catalyst worked effectively for ketones with a lithium(I) dicyanotrimethylsilicate(IV), as a key cyano reagent. As a result, the catalytic enantioselective cyanosilylation of ketones was successfully achieved with short reaction times and high yields combined with high enantioselectivities (Figure 8).
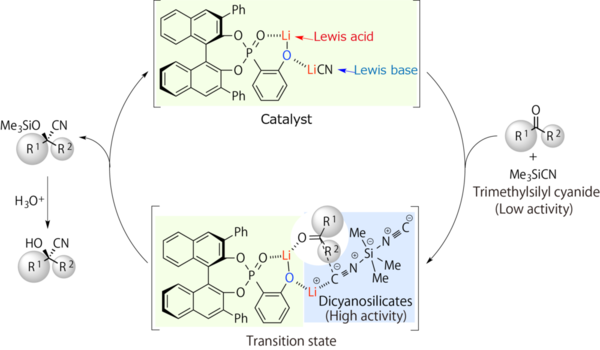 Figure 8. Possible catalytic cycles
Figure 8. Possible catalytic cycles
“Our robust catalytic system was employed in a large scale reaction.”
Prof. Ishihara and Associate Prof. Hatano address anticipate further practical use in industry, while continuing research and development. With their simple and green design of catalysts, the next step is expected to be an academia–industry collaboration.
*******
“This research field needs to achieve new records all the time, like the Olympics.”
Prof. Ishihara explained that developing catalysts will never end. Researchers are setting higher hurdles to overcome (e.g., easier processes, cheaper prices, and more eco-friendly processes to make catalysts), thereby achieving new records.
Associate Prof. Hatano mentioned that originality can be generated once the inside and out of catalysis is known. In fact, their originality in the design of catalysts is the use of lithium because it is harmless, resulting in this consistent research achievement.
For those “new” to manufacturing, the important things are;
—having the knowledge to handle fundamentals, having an abundance of ideas, and having professionality in one’s own work.
(Ayako Umemura)
Researchers featured in this article
Dr. Kazuaki Ishihara【Professor, Graduate School of Engineering, Nagoya University】
 Dr. Ishihara graduated from the Department of Applied Chemistry, School of Engineering, Nagoya University in 1981, and continued to complete his master’s course in 1987, obtaining his doctorate title in 1991. Between 1991 and 1992, he worked as a postdoctoral fellow at Harvard University. Then, on moving back to the Graduate School of Engineering at Nagoya University in 1992, he started as an assistant professor at the Department of Applied Chemistry and then in 1994, he became an assistant professor at the Department of Biotechnology. From 1997 to 2001, he worked as an associate professor for the Research Center of Waste and Emission Management, Nagoya University, and developed green chemistry. Since 2002, he has been a professor at the Department of Biotechnology, Graduate School of Engineering, Nagoya University.
Dr. Ishihara graduated from the Department of Applied Chemistry, School of Engineering, Nagoya University in 1981, and continued to complete his master’s course in 1987, obtaining his doctorate title in 1991. Between 1991 and 1992, he worked as a postdoctoral fellow at Harvard University. Then, on moving back to the Graduate School of Engineering at Nagoya University in 1992, he started as an assistant professor at the Department of Applied Chemistry and then in 1994, he became an assistant professor at the Department of Biotechnology. From 1997 to 2001, he worked as an associate professor for the Research Center of Waste and Emission Management, Nagoya University, and developed green chemistry. Since 2002, he has been a professor at the Department of Biotechnology, Graduate School of Engineering, Nagoya University.
***
Since he conducted lectures in bio-organic chemistry at the Department of Biotechnology, he obtained some hints from enzymes in creatures to design catalysts. “Enzymes circulate with economics. Even if they create by-products, another enzyme could cope with that. We can learn from such a natural system and scheme,” explained Dr. Ishihara. In fact, he is aiming for green development of catalysts like enzymes.
Dr. Ishihara is a leading professional in the molecular world. I expect further great research achievements from him (by AU)
Dr. Manabu Hatano【Associate Prof., Graduate School of Engineering, Nagoya University】
 Dr. Hatano graduated from the Chemical Engineering Department, at the Tokyo Institute of Technology in 1998, and continued to complete his master’s course in 2000. Between April 2000 and March 2003, he pursued his research during a doctoral course at Tokyo Institute of Technology working as a fellow of the Japan Society for the Promotion of Science, and obtained his Ph.D. in 2003. After that, he moved to the Graduate School of Engineering at Nagoya University and started working as an assistant professor in 2003, as a lecturer in 2007, and as an associate professor in 2012.
Dr. Hatano graduated from the Chemical Engineering Department, at the Tokyo Institute of Technology in 1998, and continued to complete his master’s course in 2000. Between April 2000 and March 2003, he pursued his research during a doctoral course at Tokyo Institute of Technology working as a fellow of the Japan Society for the Promotion of Science, and obtained his Ph.D. in 2003. After that, he moved to the Graduate School of Engineering at Nagoya University and started working as an assistant professor in 2003, as a lecturer in 2007, and as an associate professor in 2012.
(Photo: Dr. Hatano (left) and Mr. Yamakawa (right))
***
Dr. Hatano’s desk is placed inside the laboratory, so that it is convenient for him to answer questions from students immediately. The three generations of catalysts have actually been graduation works for his students and were achieved through fruitful discussions with his students. For students to challenge such unknown fields, Dr. Hatano’s mentorship must be a great encouragement.
The simple design of their catalysts makes them easy to produce; however, this is also risky as they are easy to be copied. “Here originality is required,” claimed Dr. Hatano. Teaching such professionality to his students as well, I look forward to his great success in the future (by AU)
Links
- K. Ishihara Laboratory HP http://www.ishihara-lab.net/english/
- Graduate School or Engineering HP http://www.engg.nagoya-u.ac.jp/en/index.html
- K. Ishihara Laboratory Facebook Page https://www.facebook.com/kishiharalab/
- K. Ishihara Laboratory Twitter https://twitter.com/PIodide
- Link to this article (from a search-result)
(Cover letter) http://onlinelibrary.wiley.com/doi/10.1002/anie.201601600/abstract
(Article) http://onlinelibrary.wiley.com/doi/10.1002/anie.201510682/abstract
Manabu Hatano , Katsuya Yamakawa, Tomoaki Kawai, Takahiro Horibe, and Kazuaki Ishihara.
Enantioselective Cyanosilylation of Ketones with Lithium(I) Dicyanotrimethylsilicate(IV) Catalyzed by a Chiral Lithium(I) Phosphoryl Phenoxide.
Angew. Chem. Int. Ed. 55, 4021-4025 (2016).
(First published on February 24, 2016; doi: 10.1002/anie.201601600)
- Link to related article1 (from a search-result)
http://pubs.acs.org/doi/abs/10.1021/ja051125c
Manabu Hatano , Takumi Ikeno , Takashi Miyamoto , and Kazuaki Ishihara.
Chiral Lithium Binaphtholate Aqua Complex as a Highly Effective Asymmetric Catalyst for Cyanohydrin Synthesis
J. Am. Chem. Soc. 127: 10776 (2005).
(First published on July 13, 2005; doi: 10.1021/ja051125c)
- Link to related article2 (from a search-result)
http://onlinelibrary.wiley.com/doi/10.1002/adsc.200800314/abstract
Manabu Hatano, Takumi Ikeno, Tokihiko Matsumura, Shinobu Torii, and Kazuaki Ishihara.
Chiral Lithium Salts of Phosphoric Acids as Lewis Acid–Base Conjugate Catalysts for the Enantioselective Cyanosilylation of Ketones.
Adv. Synth. Catal. 350: 1776 (2008).
(First published on July 9, 2008; doi: 10.1002/adsc.200800314)
NU Research
(English)

