Highlights
Highlights
Development of a non-invasive, quick, and low-cost system for cell quality evaluationーa step ahead to supply high quality and quantity of iPS cells
- Read in Japanese
- ツイート
- 2017/03/23
- Graduate School of Pharmaceutical Sciences
- Associate Prof. Ryuji Kato
 Dr. Ryuji Kato, an Associate Professor at the Graduate School of Pharmaceutical Sciences at Nagoya University, Japan, Dr. Miho K. Furue, the Project Leader of the Laboratory of Stem Cell Cultures at the National Institutes of Biomedical Innovation, Health and Nutrition, Japan, and Mr. Yasujiro Kiyota, the Director of Stem Cell Business Development Section at Nikon Corporation, Japan, developed a non-invasive image-based evaluation method for induced pluripotent stem cells (iPSCs) in the cultivation process.
Dr. Ryuji Kato, an Associate Professor at the Graduate School of Pharmaceutical Sciences at Nagoya University, Japan, Dr. Miho K. Furue, the Project Leader of the Laboratory of Stem Cell Cultures at the National Institutes of Biomedical Innovation, Health and Nutrition, Japan, and Mr. Yasujiro Kiyota, the Director of Stem Cell Business Development Section at Nikon Corporation, Japan, developed a non-invasive image-based evaluation method for induced pluripotent stem cells (iPSCs) in the cultivation process.
With their unlimited proliferation and multilineage potential, human pluripotent stem cells (hPSCs), such as iPSCs, are expected to apply their potentiality to the research field of drug discovery and regenerative medicine. However, at present, iPSC colonies are manually cultivated and their quality is evaluated based on their morphology by a practiced human eye, which requires substantial time and effort.
In this study, the researchers successfully designed a novel unbiased image-based analytical method, which is non-invasive, quick, and low-cost, to evaluate the quality of iPSC colonies. This system is expected to be facilitated by automated technology for the stable mass production of high quality hPSCs. The research results were published online in Scientific Reports on September 26, 2016.
This study was conducted as a part of the project focused on Developing Key Evaluation Technology: Development of Cell Production and Processing Systems for Commercialization of Regenerative Medicine (Project leader: Prof. Masahiro Kino-oka, Osaka University) supported by Japan Agency for Medical Research and Development (AMED).
This study was supported by grants-in-aid from the New Energy and Industrial Technology Development Organization (NEDO) and the Japan Agency for Medical Research and Development (AMED) to Nikon, R.K. and M.K.F.; Industrial Technology Research (Financial Support to Young Researchers, 09C46036a) to R.K.; the Japan Society for the Promotion of Science (JSPS) to R.K., M.K.F., M.S. and T.F.; the Hori Science and Arts Foundation to R.K. and H.S.; the Program for Leading Graduate Schools "Integrative Graduate Education and Research in Green Natural Sciences" of the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan to H.S.; and the Ministry of Health, Labor, and Welfare of Japan and AMED to M.K.F.
With new perspective through multidisciplinary experience and attentive discussion, researchers tackle unsolved issues.
"Technological development may cause dynamic changes in manufacturing scene."
Associate Prof. Ryuji Kato at the Graduate School of Pharmaceutical Sciences, Nagoya University, has led "manufacturing research" in the interdisciplinary fields of biology, chemistry, and informatics. Specifically, he has expertise in bioengineering to regulate manufacturing of industrial fermentation processes for products, such as bread, sake, and biological medicine. "Living creatures are difficult to control; therefore, technological development is required to assist those involved in manufacturing," says Associate Prof. Kato.
More factors may influence the final products during biological manufacturing than chemical manufacturing. Since the scale of biological manufacturing is large and the procedure is complex, much information obtained from monitoring and measurement must be controlled throughout the process. For the industrial biological production, Associate Prof. Kato has addressed real-time monitoring through compiling a database and developing computer models.
The research group in this study aimed to validate the utility of automated quantitative evaluation method in hPSCs, such as iPSCs, for cell quality control. Using non-invasive image-based evaluation technology, they successfully developed algorithm for a new software by handling a mass of image data of colonies in size and morphology. After developing a non-invasive, quick, and low-cost system for cell quality evaluation, they have endeavored to find the detailed functions of biological mechanism in iPSCs.
*******
—An issue of manual evaluation process on iPSC cultures
 In drug discovery research, hPSCs are highly expected to be useful in finding the cause of diseases, evaluation of new drugs, and so on. The discovery of human embryonic stem cells (hESCs) in 1998 and human iPSCs (hiPSCs) in 2006 enabled researchers in life science to utilize any type of human cells in any amount to develop potential medicinal applications.
In drug discovery research, hPSCs are highly expected to be useful in finding the cause of diseases, evaluation of new drugs, and so on. The discovery of human embryonic stem cells (hESCs) in 1998 and human iPSCs (hiPSCs) in 2006 enabled researchers in life science to utilize any type of human cells in any amount to develop potential medicinal applications.
hPSCs have two main abilities: the potential to differentiate into various types of tissues/organs, and an infinite proliferation ability. Therefore, the technology to use hPSCs enables to produce multiple types of cells as many as researchers require: the hPSC technology can examine the effectiveness and side-effects of unknown medicines, which are difficult to study directly on humans.
“All cells have been manually produced. Henceforth, large quantities of cells will be required for future research of drug delivery systems.”
Currently, the cultivation of cells is performed manually, and the quality of cells is also manually evaluated by the morphological observation under microscopy. In this evaluation process, colonies with “strange morphology” are carefully scraped off from the cultivation vessel, in order to keep undifferentiated hPSCs only in culture. Associate Prof. Kato claims that technological innovations, including mechanization, are essential to assist researchers in the evaluation method.
—A challenge to the automation of the evaluation method with image-based analytical technology
“On being asked by Prof. Kino-oka at AMED project, this study began because the project required engineering perspective.”
Prof. Masahiro Kino-oka at Osaka University, who leads the AMED project, has developed manufacturing technology for industrialization in the field of regenerative medicine by collaborating with many companies. An example of his accomplishments is automation as in his established concept, specifically for regenerative therapies to achieve an optimal balance between efficiency and cost of production.
Tissue Factory (T-Factory) PV_English
Associate Prof. Kato participated in this project to develop algorithm for a new software by image-based classification of undifferentiated/differentiated iPSC colonies for the automatic quality control. The research group assigned Dr. Miho K. Furue, the Project Leader of the Laboratory of Stem Cell Cultures, National Institutes of Biomedical Innovation, Health and Nutrition, Japan, who has been leading the morphological quality control of stem cells, and Mr. Yasujiro Kiyota, the Manager of Stem Cell Business Development Section from Nikon Corporation, Japan, who has developed automated cell observation system, BioStation CT. Together, they started developing a new evaluation method to meet the needs in the evaluation technology of iPSCs.
—To record all variations in heterogenic iPSC colonies
Under a common culture condition, ESCs and iPSCs form colonies in a culture vessel. Because a colony is the aggregation of 2-3,000 two-dimensional cells derived from a single parent cell, the morphology of colony exhibits various features, and many researchers have discussed that the morphological information is an important signature that reflects the condition of cell colonies (Figure 1). For example, a round colony indicates the undifferentiated condition of hPSCs throughout its expansion process; otherwise, cells are to change the condition into differentiated. The problem is that the cell colonies have been assessed by a human eye, which may lead to errors and instability for the evaluation.
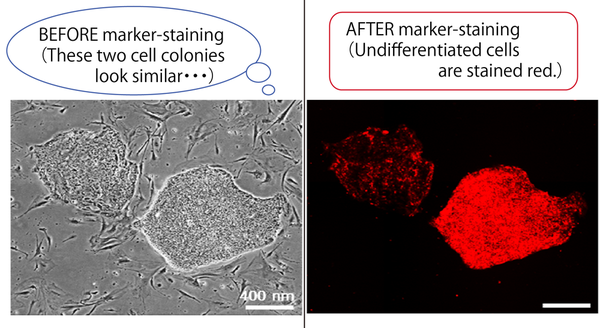 Figure 1. An example of cell colony morphologies and their conditions clarified by a marker to stain undifferentiated cells (rBC2LCN). (Figure: by courtesy of Associate Prof. Kato)
Figure 1. An example of cell colony morphologies and their conditions clarified by a marker to stain undifferentiated cells (rBC2LCN). (Figure: by courtesy of Associate Prof. Kato)
"The technology we applied to resolve this issue is similar to the technology that distinguishes people's faces."
To distinguish some appearance, "different features" together with "common features" have to be found from massive amounts of image data. The research team was challenged to develop similar algorithm to the image-based analysis of hiPSC colonies. "The essential concept was to record thoroughly all varieties of colonies, and to analyze the image data of biological information in bioinformatics," explained Associate Prof. Kato.
Listed below is the proposed methodology for the evaluation analysis of hPSC colonies. (Figures below: by courtesy of Associate Prof. Kato):
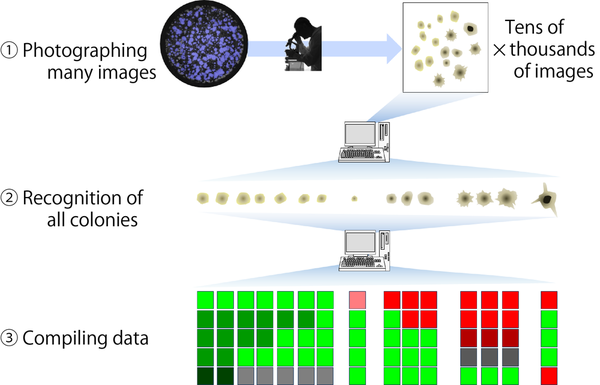
1. Massive amounts of unbiased image data of iPSC colonies are collected.
2. All colonies in the images are recognized by image processing.
3. Multiple parameters for the measurement of colony (such as the roughness of contour, the size of area, diameter, and width) are extracted from images for database-compiling.
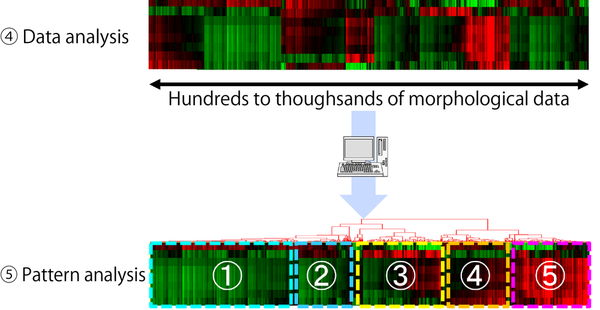
4. Morphological data of colonies are analyzed by clustering.
5. By the clustering analysis, the colonies are classified into categories. The representative morphological patterns in each category are used as the “fingerprint of colony morphology” to quantitatively describe the colony morphology.
“I followed the manner that people remember “representative colonies” under microscopy.”
Associate Prof. Kato consequently mentioned that “When we collect sufficient size of data that include various morphologies of colonies and their biological conditions, the morphological types can quantitatively be determined, indicating their biological conditions.” A breakthrough in this study was the collaboration of various technologies: morphological analysis of several colonies performed by Nikon’s system and data analysis by a bioengineering approach. Ambiguous morphological features of cells were finally distinguished owing to the computer-assisted objective analysis.
Moreover, to confirm the utility of this technology, the research group examined the global gene expression profiles with both “good- and bad-morphology” of iPSC colonies. Consequently, the bad-morphology of colonies exhibited distinctly different gene expression profiles compared with that of good-shaped ones (Figure 2).
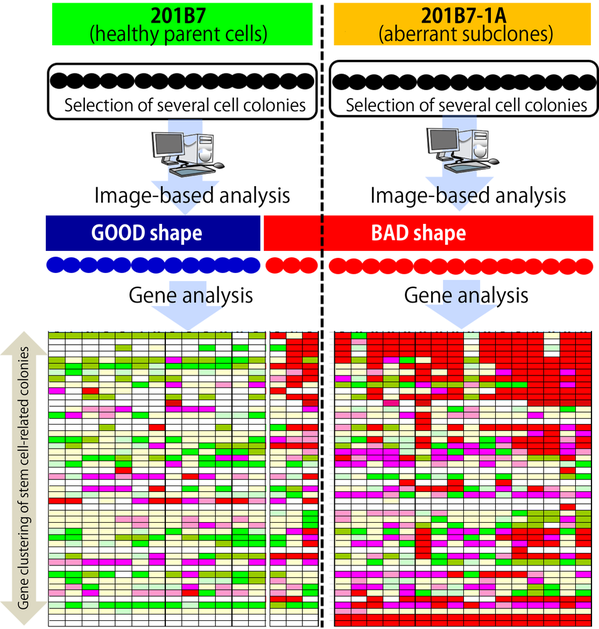 Figure 2. To examine the accuracy of the image-based analysis of iPSC colonies, the resulting colony morphologies were compared based on global gene expression profiles of individual colonies. (Figure: by courtesy of Associate Prof. Kato)
Figure 2. To examine the accuracy of the image-based analysis of iPSC colonies, the resulting colony morphologies were compared based on global gene expression profiles of individual colonies. (Figure: by courtesy of Associate Prof. Kato)
“The technological advances of image-based analysis enabled us to evaluate the quality of living cells without damaging them.”
By developing algorithm for the software of image-based analysis, the research team discovered the means of utilizing the automated evaluation of living iPSCs in the cell quality control technology. Similarly, this method could be applied for other iPSC cultures and hESCs. “We believe that the accuracy of image-based analysis will be further improved with evolved data compilation and algorithms,” expects Associate Prof. Kato.
*******
Research results have to be produced steadily.
If the cell quality control is performed easily both before and after the experiments, the production line for iPSCs can be systemized so that a large quantity of iPSCs can be economically and constantly supplied. The use of non-invasion, quick, and low-cost system is significant for cell quality evaluation; particularly, the balance between cell quality and production cost cannot be neglected in the field of regenerative medicine.
“We would be disappointed if sweet strawberries are not sweet when eating. Improvement is always necessary.”
Associate Prof. Kato added that “This is similar to the sweetness check of fruits improved by technological measurement and accuracy of evaluation.” He expects further development to advance cell production technology from the engineering point of view.
Cells, both in strawberries and humans, are interesting, important, and worth challenging in research because they are diverse in the “bio-world.”
(Ayako Umemura)
NU Researcher, featured in this article
Dr. Ryuji Kato (Associate Professor, Graduate School of Pharmaceutical Sciences, Nagoya University)
 Dr. Kato graduated from the Department of Applied Chemistry, Chemical Engineering, and Biomolecular Engineering, School of Engineering, Tohoku University, Japan, in 1999. Subsequently, he obtained his master’s degree in 2001 at the Graduate School of Biological Sciences at Nara Institute of Science and Technology, Japan. In 2004, he obtained his doctorate degree at the Graduate School of Engineering, Nagoya University, Japan. After working as a post-doctoral fellow at the Department of Biotechnology, School of Engineering at Nagoya University, he began working as an assistant professor at the Department of Cell Therapy, School of Medicine, Nagoya University in 2004, and subsequently at the Department of Biotechnology, Graduate School of Engineering, Nagoya University in 2006. Since 2012, he has been an associate professor at the Graduate School of Pharmaceutical Sciences.
Dr. Kato graduated from the Department of Applied Chemistry, Chemical Engineering, and Biomolecular Engineering, School of Engineering, Tohoku University, Japan, in 1999. Subsequently, he obtained his master’s degree in 2001 at the Graduate School of Biological Sciences at Nara Institute of Science and Technology, Japan. In 2004, he obtained his doctorate degree at the Graduate School of Engineering, Nagoya University, Japan. After working as a post-doctoral fellow at the Department of Biotechnology, School of Engineering at Nagoya University, he began working as an assistant professor at the Department of Cell Therapy, School of Medicine, Nagoya University in 2004, and subsequently at the Department of Biotechnology, Graduate School of Engineering, Nagoya University in 2006. Since 2012, he has been an associate professor at the Graduate School of Pharmaceutical Sciences.
***
Dr. Kato leads “manufacturing research.” With his multidisciplinary research experience, he pursues interdisciplinary research in biology, chemistry, and informatics to address the current issues in the fields. “The important thing is to have attentive and respective discussions with collaborative researchers,” Dr. Kato added.
The Graduate School of Pharmaceutical Sciences provides “sciences to know, create, and explore,” “brand-new ideas born from interdisciplinary research programs,” and “original and world-leading pharmaceutical research.” Dr. Kato is a representative at the Graduate School and I expect further great research achievements from him (by AU)
Links
- Laboratory of Cell and Molecular Bioengineering HP http://www.ps.nagoya-u.ac.jp/lab_pages/CMB/index-e.html
- Graduate School of Pharmaceutical Sciences HP http://www.ps.nagoya-u.ac.jp/en/
- Link to this article (from a search-result)
http://www.nature.com/articles/srep34009
Ryuji Kato, Megumi Matsumoto, Hiroto Sasaki, Risako Joto, Mai Okada, Yurika Ikeda, Kei Kanie, Mika Suga, Masaki Kinehara, Kana Yanagihara, Yujung Liu, Kozue Uchino-Yamada, Takayuki Fukuda, Hiroaki Kii, Takayuki Uozumi, Hiroyuki Honda, Yasujiro Kiyota, and Miho K Furue.
Parametric analysis of colony morphology of non-labelled live human pluripotent stem cells for cell quality control
Scientific Reports 6: 34009 (2016).
(First published on September 26, 2016; doi: 10.1038/srep34009)
NU Research
(English)